|
|
| Untersuchte Arbeit: Seite: 26, Zeilen: 1-9, 14-18 |
Quelle: Lang et al 2006 Seite(n): 1152, 1160, Zeilen: 1152: 3ff, l.col: last paragraph; 1160: figure |
|---|---|
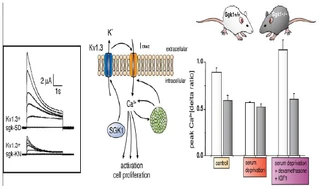
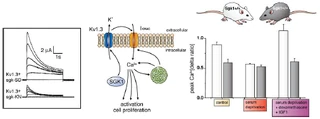
Figure nr. 4 - Middle: putative role of SGK1 in the regulation of K+ and Ca2+ channels required for stimulation of cell proliferation. Mitogenic factors stimulate the Ca2+ release-activated Ca2+ channel ICRAC. Ca2+ entry through this channel is highly sensitive to cell membrane potential, which is maintained by K+ channels. The stimulation of the voltage-sensitive K+ channel Kv1.3 by SGK1 may serve to maintain the cell membrane polarization and thus sustain oscillating Ca2+ entry through ICRAC. Left: current generated by depolarization of HEK cells overexpressing constitutively active S422DSGK1 (sgk-SD) or the inactive K127NSGK1 mutant (sgk-KN). Right: peak Ca2+ concentration after Ca2+ entry in Ca2+-depleted fibroblasts from wild-type mice (sgk1+/+, open bars) or SGK1 knockout mice (sgk1-/-, solid bars) fibroblasts. Ca2+ entry in sgk1-/- fibroblasts is not sensitive to serum deprivation or to dexamethasone plus IGF-I (Shumilina E. et al, (2005) J Cell Physiol). A common SGK1 gene variant is associated with increased blood pressure and body weight. SGK1 may thus contribute to metabolic syndrome. SGK1 may further participate in tumor growth, neurodegeneration, fibrosing disease, and the sequelae of ischemia. SGK3 is required for adequate hair growth and maintenance of intestinal nutrient transport and influences locomotive behavior. In conclusion, SGK1 cover a wide variety of physiological functions and may play an active role in a multitude of pathophysiological conditions. There is little doubt that further targets will be identified that is modulated by the SGK and that further SGK-dependent in vivo physiological functions and pathophysiological conditions will be defined. [...] SGK3 is expressed in all tissues tested thus far and is particularly high in the embryo, adult heart and spleen. Expression of SGK2 is most abundant in epithelial tissues including kidney, liver, pancreas, and presumably choroid plexus of the brain. The subcellular distribution may be nuclear and cytoplasmic, as SGK2 and SGK3 contain a similar nuclear localization signal sequence as SGK1. |
A common (~5% prevalence) SGK1 gene variant is associated with increased blood pressure and body weight. SGK1 may thus contribute to metabolic syndrome. SGK1 may further participate in tumor growth, neurodegeneration, fibrosing disease, and the sequelae of ischemia. SGK3 is required for adequate hair growth and maintenance of intestinal nutrient transport and influences locomotive behavior. In conclusion, the SGKs cover a wide variety of physiological functions and may play an active role in a multitude of pathophysiological conditions. There is little doubt that further targets will be identified that are modulated by the SGK isoforms and that further SGK-dependent in vivo physiological functions and pathophysiological conditions will be defined.
[...] SGK3 is expressed in all tissues tested thus far (172) and is particularly high in the embryo (152, 193) and adult heart and spleen (172). Expression of SGK2 is most abundant in epithelial tissues including kidney, liver, pancreas, and presumably choroid plexus of the brain (172). The subcellular distribution may be nuclear and cytoplasmic, as SGK2 and SGK3 contain a similar nuclear localization signal sequence as SGK1 (112). [page 1160] FIG. 3. Middle: putative role of SGK1 in the regulation of K+ and Ca2+ channels required for stimulation of cell proliferation. Mitogenic factors stimulate the Ca2+ release-activated Ca2+ channel ICRAC. Ca2+ entry through this channel is highly sensitive to cell membrane potential, which is maintained by K+ channels. The stimulation of the voltage-sensitive K+ channel Kv1.3 by SGK1 may serve to maintain the cell membrane polarization and thus sustain oscillating Ca2+ entry through ICRAC. Left: current generated by depolarization of HEK cells overexpressing constitutively active S422DSGK1 (sgk-SD) or the inactive K127NSGK1 mutant (sgk-KN). Right: peak Ca2+ concentration after Ca2+ entry in Ca2+-depleted fibroblasts from wild-type mice (sgk1+/+, open bars) or SGK1 knockout mice (sgk1-/-, solid bars) fibroblasts. Ca2+ entry in sgk1-/- fibroblasts is not sensitive to serum deprivation or to dexamethasone + IGF-I. [Data modified from Shumilina et al. (293).] 112. Firestone GL, Giampaolo JR, and O’Keeffe BA. Stimulus-dependent regulation of the serum and glucocorticoid inducible protein kinase (Sgk) transcription, subcellular localization and enzymatic activity. Cell Physiol Biochem 13: 1–12, 2003. 152. Huber SM, Friedrich B, Klingel K, Lenka N, Hescheler J, and Lang F. Protein and mRNA expression of serum and glucocorticoid-dependent kinase 1 in metanephrogenesis. Dev Dyn 221: 464–469, 2001. 172. Kobayashi T, Deak M, Morrice N, and Cohen P. Characterization of the structure and regulation of two novel isoforms of serumand glucocorticoid-induced protein kinase. Biochem J 344: 189–197, 1999. 193. Lee E, Lein ES, and Firestone GL. Tissue-specific expression of the transcriptionally regulated serum and glucocorticoid-inducible protein kinase (Sgk) during mouse embryogenesis. Mech Dev 103: 177–181, 2001. 293. Shumilina E, Lampert A, Lupescu A, Myssina S, Strutz-Seebohm N, Henke G, Grahammer F, Wulff P, Kuhl D, and Lang F. Deranged Kv channel regulation in fibroblasts from mice lacking the serum and glucocorticoid inducible kinase SGK1. J Cell Physiol 204: 87–98, 2005. |
The source is not given. Note that the paper Shumilina E. et al, (2005) does not contain the figure and its caption. |
|