|
|
| Untersuchte Arbeit: Seite: 33, Zeilen: figure+caption |
Quelle: Lang et al 2006 Seite(n): 1154, Zeilen: figure |
|---|---|
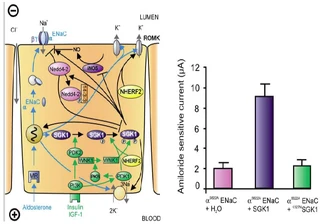
Figure nr. 6 - Left: model for the Serum- and Glucocorticoid-inducible Kinase-1 (SGK1)-dependent regulation of Na+ reabsorption and K+ secretion in the aldosterone-sensitive distal nephron. Aldosterone binds to mineralocorticoid receptors (MR) and stimulates the expression of SGK1, α-epithelial Na+ channel (αENaC), renal outer medullary K+ channel (ROMK), and the Na+-K+-ATPase. αENaC associates with constitutive β- and γ-subunits to form fully active ENaC. SGK1 can be phosphorylated on 422Ser by insulin or insulin-like growth factor I (IGF-I) through a signaling cascade involving phosphatidylinositol 3-kinase (PI3K) and an unknown kinase (PDK2?/hydrophobic motif kinase). Phosphorylated 422Ser allows binding of PDK1 and/or NHERF2 with subsequent phosphorylation of SGK1 at 256Thr. PDK1 might activate SGK1 indirectly through phosphorylation of WNK1 kinase. The mechanism of SGK1 activation by WNK1 is yet unknown but does not require SGK1 phosphorylation. Activated SGK1 increases Na+ reabsorption in part by phosphorylation of the ubiquitin ligase Nedd4–2, allowing binding of the chaperone 14–3-3 to phosphorylated 444Ser. This interaction prevents Nedd4–2-mediated ubiquitination of the ENaC-PY motif and thus internalization and degradation of ENaC. SGK1 further stimulates ENaC by upregulation of transcription, by direct phosphorylation of the channel protein and by inhibition of the inducible nitric oxide synthase (iNOS). In addition to its effect on ENaC, SGK1 stimulates the Na+-K+-ATPase and K+ channels including ROMK. Right: arithmetic means ± SE of ENaC-induced currents in Xenopus oocytes coexpression experiments showing that coexpression of wild-type SGK1 but not of the inactive mutant K127NSGK1 leads to stimulation of an ENaC mutant lacking the SGK1 phosphorylation consensus sequence (S622AENaC). |
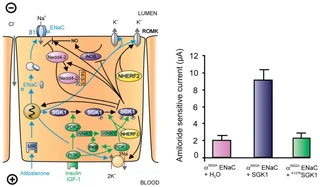
FIG. 1. Left: model for the Serum- and Glucocorticoid-inducible Kinase-1 (SGK1)-dependent regulation of Na+ reabsorption and K+ secretion in the aldosterone-sensitive distal nephron. Aldosterone binds to mineralocorticoid receptors (MR) and stimulates the expression of SGK1, α-epithelial Na+ channel (αENaC), renal outer medullary K+ channel (ROMK), and the Na+-K+-ATPase. αENaC associates with constitutive β- and γ-subunits to form fully active ENaC. SGK1 can be phosphorylated on 422Ser by insulin or insulin-like growth factor I (IGF-I) through a signaling cascade involving phosphatidylinositol 3-kinase (PI 3K) and an unknown kinase (PDK2?/hydrophobic motif kinase). Phosphorylated 422Ser allows binding of PDK1 and/or NHERF2 with subsequent phosphorylation of SGK1 at 256Thr. PDK1 might activate SGK1 indirectly through phosphorylation of WNK1 kinase. Phosphorylated 422Ser allows binding of PDK1 and/or NHERF2 with subsequent phosphorylation of SGK1 at 256Thr. PDK1 might activate SGK1 indirectly through phosphorylation of WNK1 kinase. The mechanism of SGK1 activation by WNK1 is yet unknown but does not require SGK1 phosphorylation. Activated SGK1 increases Na+ reabsorption in part by phosphorylation of the ubiquitin ligase Nedd4–2, allowing binding of the chaperone 14–3-3 to phosphorylated 444Ser. This interaction prevents Nedd4–2-mediated ubiquitination of the ENaC-PY motif and thus internalization and degradation of ENaC. SGK1 further stimulates ENaC by upregulation of transcription, by direct phosphorylation of the channel protein, and by inhibition of the inducible nitric oxide synthase (iNOS). In addition to its effect on ENaC, SGK1 stimulates the Na+-K+-ATPase and K+ channels including ROMK. Right: arithmetic means ± SE of ENaC-induced currents in Xenopus oocyte coexpression experiments showing that coexpression of wild-type SGK1 but not of the inactive mutant K127NSGK1 leads to stimulation of an ENaC mutant lacking the SGK1 phosphorylation consensus sequence (S622AENaC). |
The source is not given. |
|