58 gesichtete, geschützte Fragmente: Plagiat
| [1.] Dsa/Fragment 043 01 - Diskussion Bearbeitet: 27. August 2016, 21:21 WiseWoman Erstellt: 20. August 2016, 22:17 (WiseWoman) | Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Saparov 2007, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 43, Zeilen: 1ff (entire page) |
Quelle: Saparov 2007 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| [Figure 11]
Figure nr. 11 - The seven TRP subfamilies. Representatives of the different subfamilies are indicated at the top and bottom, respectively. Several domains are indicated: ankyrin repeats (A), coiled coil domain (cc), protein kinase domain (TRPM6/7 only), transmembrane segments, and the TRP domain.
[Figure 12] Figure nr. 12 - The quaternary structure of TRP channels allows homo- or heteromeric configurations. Left: TRP channel subunit, right: structure of functional TRP channel. |
[Representatives of the seven TRP subfamilies]
The seven TRP subfamilies. Representatives of the different subfamilies are indicated at the top and bottom, respectively. Several domains are indicated: ankyrin repeats (A), coiled coil domain (cc), protein kinase domain (TRPM6/7 only), transmembrane segments, and the TRP domain (1) TRP proteins are supposed to form 6 membrane-spanning segments whereas the pore region is formed by a hydrophilic region between S5 and S6 forms. The N- and C-termini are located intracellularly. The N-terminus often contains ankyrin repeats as well as a coiled-coil domain, which are suspected to bind with other proteins or the cytoskeleton. Especially they are thought to be needed for TRP protein interaction because a functional channel contains 4 TRP subunits, either similar or different ones, to form homo- or heteromers (4,5). [Scheme: Quaternary structure of TRP channels] The quartenary structure of TRP channels allows homo- or heteromeric configurations. Left: TRP channel subunit, right: structure of functional TRP channel 1. Montell, C. (2005) Sci STKE 2005, re3 4. Goel, M., Sinkins, W. G., and Schilling, W. P. (2002) J Biol Chem 277, 48303-48310 5. Hoenderop, J. G., Voets, T., Hoefs, S., Weidema, F., Prenen, J., Nilius, B., and Bindels, R. J. (2003) Embo J 22, 776-785 |
The source is not given. The illustrations are no longer available online. |
|
| [2.] Dsa/Fragment 042 01 - Diskussion Bearbeitet: 27. August 2016, 21:21 WiseWoman Erstellt: 20. August 2016, 22:01 (WiseWoman) | Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Saparov 2007, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 42, Zeilen: 1ff (entire page) |
Quelle: Saparov 2007 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| Transient receptor potential channels (TRPs)
The mammalian TRP channels encode a family of about 30 ion channel proteins. This superfamily consists of seven diverse groups structurally similar to the originally found Drosophila TRP and they differ in ion selectivities, modes of activation and physiological functions.
Figure nr. 10 - TRP family with its subgroups. The vanilloids, the classical, the melastatin, the mucolipins (TRPML) and the polycystins (TRPP) (members of TRPN, TRPA not shown). TRP proteins are expressed predominantly in the nervous system and are of particular importance in sensory physiology. (Montell C. et al., (2005) Sci STKE). In each subfamily are three to eight members. (Hönderop JG. et al., (2005) Physiol Rev). The reason to identify mammalian TRPs is to characterize those channels that might account for highly Ca2+ selective Ca2+ entry mechanism in nonexcitable cells, referred to as store-operated Ca2+ entry (SOCE). SOCE is interesting, due to association of these modes of Ca2+ entry with processes ranging from T cell activation to apoptosis, cell proliferation, fluid secretion and cell migration.(Montell C. et al., (2005) Sci STKE.). The TRP superfamily can be divided into two structurally different groups (Clapham DE. et al., (2003) Pharmacol Rev): • Group 1: TRPC, TRPV, TRPM, TRPN, TRPA. They share substantial sequence identity in the transmembrane domains. • Group 2: TRPP and TRPML. They have low sequence similarity and a large extracellular loop between the first and the second transmembrane domains. |
Transient receptor potential channels (TRPs)
The mammalian TRP channels encode a family of about 30 ion channel proteins. This superfamily consists of seven diverse groups structurally similar to the originally found Drosophila TRP and they differ in ion selectivities, modes of activation and physiological functions. TRP proteins are expressed predominantly in the nervous system and are of particular importance in sensory physiology. (1) [Illustration] Summary of the different TRP family members TRP family with its subgroups: the vanilloids, the classical, the melastatin, the mucolipins (TRPML) and the polycystins (TRPP) (members of TRPN, TRPA not shown). In each subfamily are three to eight members. (2) The reason to identify mammalian TRPs is to characterize those channels that might account for highly Ca2+ selective Ca2+ entry mechanism in nonexcitable cells, referred to as store-operated Ca2+ entry (SOCE). SOCE is interesting, due to association of these modes of Ca2+ entry with processes ranging from T cell activation to apoptosis, cell proliferation, fluid secretion and cell migration.(1) The TRP superfamily can be divided into two structurally different groups(1,3): 1. Group 1: TRPC, TRPV, TRPM, TRPN, TRPA They share substantial sequence identity in the transmembrane domains. 2. Group 2: TRPP and TRPML They have low sequence similarity and a large extracellular loop between the first and the second transmembrane domains. 1. Montell, C. (2005) Sci STKE 2005, re3 2. Hoenderop, J. G., Nilius, B., and Bindels, R. J. (2005) Physiol Rev 85, 373-42 3. Clapham, D. E., Montell, C., Schultz, G., and Julius, D. (2003) Pharmacol Rev 55, 591-596 |
The source is not given |
|
| [3.] Dsa/Fragment 044 01 - Diskussion Bearbeitet: 27. August 2016, 21:16 WiseWoman Erstellt: 20. August 2016, 22:25 (WiseWoman) | Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Saparov 2007, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 44, Zeilen: 1-14 |
Quelle: Saparov 2007 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| TRPV (vanilloid) family has six mammalian members grouped into three subfamilies. These proteins contain three to five ankyrin repeats and share ~25% amino acid identity to TRPC proteins (Montell C. et al, (2005) Sci STKE). TRPV1-TRPV4 form poor selective cation channels and are sensitive to heat (Hellwig N. et al., (2005) J Cell Sci). TRPV5 and TRPV6 are phylogenetically closely related Ca2+ selective channels (PCa: PNa > 100) expressed in epithelia of kidney and intestine and exhibit a constitutive activity (Clapham DE. et al., (2003) Pharmacol Rev). Both proteins become permeable to monovalent cations in the absence of divalent cations. It was proposed that TRPV6 may be the highly Ca2+-selective, store-operated channels, referred to CRAC but several biophysical properties of TRPV6 are distinct from those of ICRAC (Kahr H. et al., (2004) J Physiol). Nevertheless there remains still the option that TRPV5/ TRPV6 may be subunits of CRAC channels.
TRPM (long TRPC, melastatin) family is composed of eight members. They share ~20% identity, have a TRP domain and contain ankyrin repeats at the N-terminus which is longer than that of TRPCs and TRPVs (Fleig A. et al., (2004) Novartis Found Symp). |
TRPV (vanilloid) family has six mammalian members grouped into three subfamilies. These proteins contain three to five ankyrin repeats and share ~25% amino acid identity to TRPC proteins (1). TRPV1-TRPV4 form poor selective cation channels and are sensitive to heat (6,7). TRPV5 and TRPV6 are phylogenetically closely related Ca2+ selective channels (PCa:PNa > 100) expressed in epithelia of kidney and intestine and exhibit a constitutive activity. (3) Both proteins become permeable to monovalent cations in the absence of divalent cations. It was proposed that TRPV6 may be the highly Ca2+-selective, store-operated channels, referred to CRAC but several biophysical properties of TRPV6 are distinct from those of ICRAC (8). Nevertheless there remains still the option that TRPV5/ TRPV6 may be subunits of CRAC channels.(1)
TRPM (long TRPC, melastatin) family is composed of eight members. They share ~20% identity , have a TRP domain and contain ankyrin repeats at the N-terminus which is longer than that of TRPCs and TRPVs. (9) 1. Montell, C. (2005) Sci STKE 2005, re3 3. Clapham, D. E., Montell, C., Schultz, G., and Julius, D. (2003) Pharmacol Rev 55, 591-596 6. Hellwig, N., Albrecht, N., Harteneck, C., Schultz, G., and Schaefer, M. (2005) J Cell Sci 118, 917-928 7. Wang, H., and Woolf, C. J. (2005) Neuron 46, 9-12 8. Kahr, H., Schindl, R., Fritsch, R., Heinze, B., Hofbauer, M., Hack, M. E., Mortelmaier, M. A., Groschner, K., Peng, J. B., Takanaga, H., Hediger, M. A., and Romanin, C. (2004) J Physiol 557, 121-132 9. Fleig, A., and Penner, R. (2004) Novartis Found Symp 258, 248-258; discussion 258-266 |
The source is not given. |
|
| [4.] Dsa/Fragment 042 00 - Diskussion Bearbeitet: 27. August 2016, 21:14 WiseWoman Erstellt: 18. May 2015, 16:53 (Hindemith) | Dsa, Fragment, Gesichtet, Hoenderop et al 2005, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 42, Zeilen: figure |
Quelle: Hoenderop et al 2005 Seite(n): 1 (online source), Zeilen: figure |
|---|---|
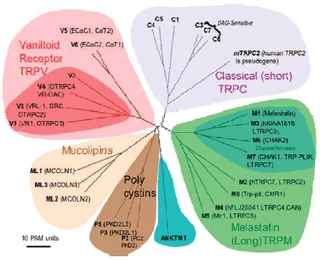
Figure nr. 10 - TRP family with its subgroups. The vanilloids, the classical, the melastatin, the mucolipins (TRPML) and the polycystins (TRPP) (members of TRPN, TRPA not shown). |
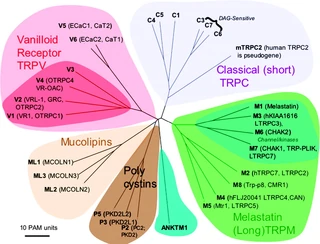
Fig. 2. Mammalian TRP family tree. The evolutionary distance between the TRP channels is shown by the total branch lengths in point accepted mutations (PAM) units, which is the mean number of substitutions per 100 residues. The tree was calculated using the neighbor-joining method for human, rat, and mouse sequences. [From Clapham (74).] |
Saparov 2007 is the source for the entire page, but it is only available in the Internet Archives without the illustrations. Saparov gives Hoenderop et al 2005 as the source for this illustration, and it is identical, although the caption in Dsa is the caption from Saparov 2007 and not the caption from Hoenderop et al 2005. |
|
| [5.] Dsa/Fragment 043 00 - Diskussion Bearbeitet: 26. August 2016, 20:45 Hindemith Erstellt: 21. August 2016, 13:08 (WiseWoman) | Dsa, Fragment, Gesichtet, Montell 2005, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 43, Zeilen: Figure 11 |
Quelle: Montell 2005 Seite(n): 2, Zeilen: Figure 1 |
|---|---|
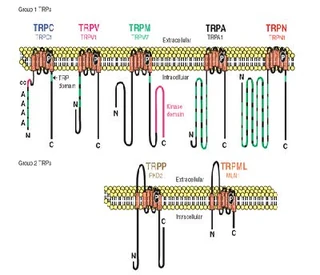
Figure nr. 11 - The seven TRP subfamilies. Representatives of the different subfamilies are indicated at the top and bottom, respectively. Several domains are indicated: ankyrin repeats (A), coiled coil domain (cc), protein kinase domain (TRPM6/7 only), transmembrane segments, and the TRP domain. |
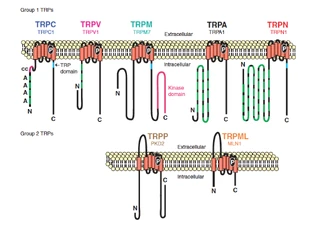
Fig. 1. The seven TRP subfamilies. Representatives of the five group 1 and two group 2 subfamilies are indicated at the top and bottom, respectively. Several domains are indicated: ankyrin repeats (A), coiled coil domain (cc), protein kinase domain (TRPM6/7 only), transmembrane segments, and the TRP domain (see Fig. 2). |
The text surrounding this picture is taken from Quelle:Dsa/Saparov_2007. The illustration is no longer available in the copy at the Internet Archive, but the reference in Saparov 2007 is to Montell 2005. Dsa has two references to Montell 2005 on the previous page, but they do not pertain to this illustration. |
|
| [6.] Dsa/Fragment 041 06 - Diskussion Bearbeitet: 20. August 2016, 19:42 WiseWoman Erstellt: 18. May 2015, 17:13 (Hindemith) | Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Van Abel et al 2005 |
|
|
| Untersuchte Arbeit: Seite: 41, Zeilen: 6-9 |
Quelle: Van Abel et al 2005 Seite(n): 1708, 1709, Zeilen: 1708: r.col: second last sentence; 1709: l.col: 4ff |
|---|---|
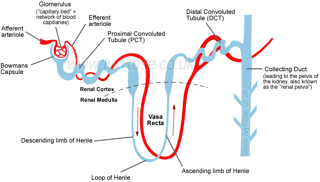
| Active Ca2+ reabsorption takes place in the distal convoluted tubule (DCT) and connecting tubule (CNT) of the kidney. PTH receptors have been detected throughout the kidney, as well as in the actively Ca2+ transporting tubules DCT and CNT. | Active Ca2+ reabsorption takes place in the distal convoluted tubule (DCT) and connecting tubule (CNT) of the kidney. [...]
[page 1709] [...] PTH receptors have been detected throughout the kidney, as well as in the actively Ca2+ transporting tubules DCT and CNT [9, 10]. 9. RICCARDI D, LEE WS, LEE K, et al: Localization of the extracellular Ca2+-sensing receptor and PTH/PTHrP receptor in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 271:F951–F956, 1996 10. YANG TX, HASSAN S, HUANG YG, et al: Expression of PTHrP, PTH/PTHrP receptor, and Ca2+-sensing receptor mRNAs along the rat nephron. Am J Physiol 272:F751–F758, 1997 |
No source is mentioned. |
|
| [7.] Dsa/Fragment 056 00 - Diskussion Bearbeitet: 20. August 2016, 19:30 WiseWoman Erstellt: 18. May 2015, 15:42 (Hindemith) | Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Wulff et al 2002 |
|
|
| Untersuchte Arbeit: Seite: 56, Zeilen: figure |
Quelle: Wulff et al 2002 Seite(n): 1264, Zeilen: figure |
|---|---|
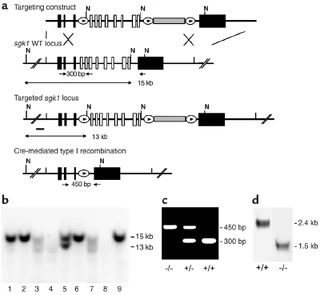
Figure nr. 13 - Generation of sgk1–/– mice. (a) Targeting strategy. The neomycin resistance cassette (gray box) was flanked by two loxP sites (ovals) and inserted into intron 11. Exons 4–11, which code for the Sgk1 kinase domain (open boxes), were “floxed” by inserting a third loxP site into intron 3. N indicates NheI restriction sites, and the small black bar indicates the external 5′ probe used for Southern blot analysis. Expected fragment sizes of the wild-type and targeted sgk1 locus are also indicated. One homologously recombined ES cell clone was transiently transfected with Cre recombinase, and a clone that had undergone recombination between the first and the third loxP site (type I recombination) was chosen for injection. Arrows below the gene indicate PCR primers used for genotyping. Numbers between the arrows indicate the size of the amplified fragments. Crossed bars below (a) indicate homologous recombination. (b) Southern blot of NheI-digested genomic DNA from ES cell clones after gene targeting hybridized with a 5′ external probe (black bar in a). Lane 5 shows a targeted ES cell line. (c) Genotyping by PCR of genomic tail DNA of homozygous (–/–) and heterozygous (-/+) sgk1-deficient mice and wild-type mice (+/+) using a mix of three specific primers (arrows in a). (d) Autoradiograph of Northern blot analysis of Sgk1-specific transcripts in +/+ and –/– mice. The deletion of the kinase domain from the genome results in a size reduction of 0.9 kb at the mRNA level in sgk1–/– mice. |
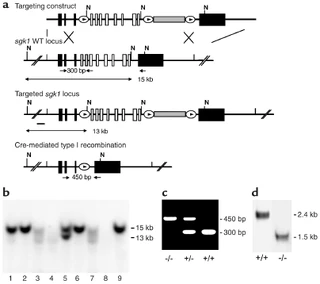
Figure 1 Generation of sgk1–/– mice. (a) Targeting strategy. The neomycin resistance cassette (gray box) was flanked by two loxP sites (ovals) and inserted into intron 11. Exons 4–11, which code for the Sgk1 kinase domain (open boxes), were “floxed” by inserting a third loxP site into intron 3. N indicates NheI restriction sites, and the small black bar indicates the external 5′ probe used for Southern blot analysis. Expected fragment sizes of the wild-type and targeted sgk1 locus are also indicated. One homologously recombined ES cell clone was transiently transfected with Cre recombinase, and a clone that had undergone recombination between the first and the third loxP site (type I recombination) was chosen for injection. Arrows below the gene indicate PCR primers used for genotyping. Numbers between the arrows indicate the size of the amplified fragments. Crossed bars below a indicate homologous recombination. (b) Southern blot of NheI-digested genomic DNA from ES cell clones after gene targeting hybridized with a 5′ external probe (black bar in a). Lane 5 shows a targeted ES cell line. (c) Genotyping by PCR of genomic tail DNA of homozygous (–/–) and heterozygous (–/+) sgk1-deficient mice and wild-type mice (+/+) using a mix of three specific primers (arrows in a). (d) Autoradiograph of Northern blot analysis of Sgk1-specific transcripts in +/+ and –/– mice. The deletion of the kinase domain from the genome results in a size reduction of 0.9 kb at the mRNA level in sgk1–/– mice. |
The source is not mentioned here. |
|
| [8.] Dsa/Fragment 022 21 - Diskussion Bearbeitet: 20. August 2016, 19:19 WiseWoman Erstellt: 18. May 2015, 15:15 (Hindemith) | BauernOpfer, BelAiba et al 2006, Dsa, Fragment, Gesichtet, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 22, Zeilen: 21-42 |
Quelle: BelAiba et al 2006 Seite(n): 828, 829, Zeilen: 828: r.col: 4ff; 829: l.col: 1ff |
|---|---|
| The serum- and glucocorticoid-inducible kinase-1, SGK1, is a known downstream effector of the PI3K cascade. SGK1 belongs to the “AGC” family of serine-threonine kinases and shares approximately 45% to 55% homology with Akt in its catalytic domain.
In contrast to Akt, SGK1 is also regulated at the transcriptional level in response to various hormones, growth factors, and extracellular stresses in a cell type – dependent manner, allowing sgk1 to be available for its targets only when needed. SGK1 was originally cloned from murine mammary tumor cells as a glucocorticoid-responsive gene. Human SGK1 was subsequently cloned as a cell volume-sensitive gene upregulated by hypertonic cell shrinkage. Increasing evidence suggests that expression, enzymatic activity, and cellular localization of SGK1 are regulated in response to various stimuli controlling not only cell volume and epithelial transport, but also cardiac action potential and cell proliferation, survival, and apoptosis. Excessive transcription of SGK1 has been shown to parallel diabetic nephropathy, glomerulonephritis, hepatic cirrhosis, pulmonary fibrosis, and polymorphisms of the SGK1 gene correlated with hypertension. Despite the wide tissue distribution of sgk1 and its sensitivity to various stimuli, the role of SGK-1 in the cardiovascular and pulmonary system remained ill defined. Because heparin, an inhibitor of thrombin formation, has been shown to decrease SGK1 mRNA in aortic smooth muscle cells, we hypothesized that SGK1 may play a role in thrombin signaling in human pulmonary artery smooth muscle cells (PASMC). We found that SGK1 is activated and induced by thrombin, that it regulates TF expression and activity in PASMC, and that it is present in remodeled pulmonary vessels with media hypertrophy associated with ph (Rachida S. et al., (2006) Circ Res). |
The serum- and glucocorticoid-inducible kinase-1, Sgk-1, is a known downstream effector of the PI3K cascade. Sgk-1 belongs to the “AGC” family of serine-threonine kinases and shares approximately 45% to 55% homology with Akt in its catalytic domain.6 In contrast to Akt, Sgk-1 is also regulated at the transcriptional level in response to various hormones, growth factors, and extracellular stresses in a cell type– dependent manner, allowing Sgk-1 to be available for its targets only when needed.7,8
Sgk-1 was originally cloned from murine mammary tumor cells as a glucocorticoid-responsive gene.6 Human Sgk-1 was subsequently cloned as a cell volume-sensitive gene upregulated by hypertonic cell shrinkage.9 Increasing evidence [page 829] suggests that expression, enzymatic activity, and cellular localization of Sgk-1 are regulated in response to various stimuli controlling not only cell volume and epithelial transport, but also cardiac action potential and cell proliferation, survival, and apoptosis.7,8 Excessive transcription of Sgk-1 has been shown to parallel diabetic nephropathy,10 glomerulonephritis,11 hepatic cirrhosis,12 pulmonary fibrosis,13 and polymorphisms of the Sgk-1 gene correlated with hypertension.14 Despite the wide tissue distribution of Sgk-1 and its sensitivity to various stimuli, the role of Sgk-1 in the cardiovascular and pulmonary system remained ill defined. Because heparin, an inhibitor of thrombin formation, has been shown to decrease Sgk-1 mRNA in aortic smooth muscle cells,15 we hypothesized that Sgk-1 may play a role in thrombin signaling in human pulmonary artery smooth muscle cells (PASMC), the main cell type involved in PH. We found that Sgk-1 is activated and induced by thrombin, that it regulates TF expression and activity in PASMC, and that it is present in remodeled pulmonary vessels with media hypertrophy associated with PH. 6. Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol Cell Biol. 1993;13:2031–2040. 7. Firestone GL, Giampaolo JR, O’Keeffe BA. Stimulus-dependent regulation of serum and glucocorticoid inducible protein kinase (SGK) transcription, subcellular localization and enzymatic activity. Cell Physiol Biochem. 2003;13:1–12. 8. Lang F, Cohen P. Regulation and physiological roles of serum- and glucocorticoid-induced protein kinase isoforms. Sci STKE. 2001; 108:RE17. 9. Waldegger S, Barth P, Raber G, Lang F. Cloning and characterization of a putative human serine/threonine protein kinase transcriptionally modified during anisotonic and isotonic alterations of cell volume. Proc Natl Acad Sci U S A. 1997;94:4440–4445. 10. Kumar JM, Brooks DP, Olson BA, Laping NJ. Sgk, a putative serine/ threonine kinase, is differentially expressed in the kidney of diabetic mice and humans. J Am Soc Nephrol. 1999;10:2488–2494. 11. Friedrich B, Warntges S, Klingel K, Sauter M, Kandolf R, Risler T, Muller GA, Witzgall R, Kriz W, Grone HJ, Lang F. Up-regulation of the human serum and glucocorticoid-dependent kinase 1 in glomerulonephritis. Kidney Blood Press Res. 2002;25:303–307. 12. Fillon S, Klingel K, Warntges S, Sauter M, Gabrysch S, Pestel S, Tanneur V, Waldegger S, Zipfel A, Viebahn R, Haussinger D, Broer S, Kandolf R, Lang F. Expression of the serine/threonine kinase hSGK1 in chronic viral hepatitis. Cell Physiol Biochem. 2002;12:47–54. 13. Waerntges S, Klingel K, Weigert C, Fillon S, Buck M, Schleicher E, Rodemann HP, Knabbe C, Kandolf R, Lang F. Excessive transcription of the human serum and glucocorticoid dependent kinase hSGK1 in lung fibrosis. Cell Physiol Biochem. 2002;12:135–142. 14. Busjahn A, Aydin A, Uhlmann R, Krasko C, Bahring S, Szelestei T, Feng Y, Dahm S, Sharma AM, Luft FC, Lang F. Serum- and glucocorticoidregulated kinase (SGK1) gene and blood pressure. Hypertension. 2002; 40:256–260. 15. Delmolino LM, Castellot JJ Jr. Heparin suppresses sgk, an early response gene in proliferating vascular smooth muscle cells. J Cell Physiol. 1997; 173:371–379. |
The reference to "Rachida S. et al., (2006) Circ Res" potentially indicates the source, as the first author of the source is named "Rachida S. BelAiba". No such entry cannot be found in the bibliography, and nothing indicates that text spanning two paragraphs has been copied from it, mostly verbatim. |
|
| [9.] Dsa/Fragment 022 02 - Diskussion Bearbeitet: 20. August 2016, 19:07 WiseWoman Erstellt: 18. May 2015, 12:07 (Hindemith) | Busjahn et al 2002, Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 22, Zeilen: 2-14 |
Quelle: Busjahn et al 2002 Seite(n): 256, Zeilen: l.col: 1ff |
|---|---|
| The serum- and glucocorticoid-regulated kinase (SGK) was originally cloned from rat mammary tumor cells as a glucocorticoid responsive gene. The human isoform was subsequently cloned as a cell volume–sensitive gene upregulated by both hypertonic and isotonic cell shrinkage (Waldegger S. et al., (1997) Proc Natl Acad Sci; Waldegger S. et al., (2000) Cell Physiol Biochem).
Because of the discovery of the 2 isoforms SGK2 and SGK3 (Kobayashi T. et al., (1999) Biochem J) the originally cloned kinase is labeled SGK1. SGK1 is expressed in renal tubular epithelial cells (Naray-Fejes-Toth A. et al., (1999) J Biol Chem; Loffing J. et al., (2001) Am J Physiol Renal Physiol) and its transcription is strongly stimulated by mineralocorticoids (Chen SY. et al., (1999) Proc Natl Acad Sci USA) suggesting a role in renal Na+ regulation. Indeed, coexpression of SGK1 with the renal epithelial Na+ channel (ENaC), in Xenopus oocytes markedly upregulates Na+ channel activity by enhancing channel protein abundance in the cell membrane. |
The serum- and glucocorticoid-regulated kinase (SGK) was originally cloned from rat mammary tumor cells as a glucocorticoid responsive gene.1 The human isoform was subsequently cloned as a cell volume–sensitive gene upregulated by both hypertonic and isotonic cell shrinkage.2,3 Because of the discovery of the 2 isoforms SGK2 and SGK3,4 the originally cloned kinase is labeled SGK1. SGK1 is expressed in renal tubular epithelial cells,5,6 and its transcription is strongly stimulated by mineralocortcoids,7 suggesting a role in renal Na+ regulation. Indeed, coexpression of SGK1 with the renal epithelial Na+ channel (ENaC) in Xenopus oocytes markedly upregulates Na+ channel activity by enhancing channel protein abundance in the cell membrane.5–9
1. Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol Cell Biol. 1993;13:2031–2040. 2. Waldegger S, Barth P, Raber G, Lang F. Cloning and characterization of a putative human serine/threonine protein kinase transcriptionally modified during anisotonic and isotonic alterations of cell volume. Proc Natl Acad Sci U S A. 1997;94:4440–4445. 3. Waldegger S, Gabrysch S, Barth P, Fillon S, Lang F. h-sgk serine threonine protein kinase as transcriptional target of p38/MAP kinase pathway in HepG2 human hepatoma cells. Cell Physiol Biochem. 2000;10:203–208. 4. Kobayashi T, Deak M, Morrice N, Cohen P. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem J. 1999;344:189–197. 5. Naray-Fejes-Toth A, Canessa C, Cleaveland ES, Aldrich G, Fejes-Toth G. Sgk is an aldosterone-induced kinase in the renal collecting duct: effects on epithelial Na+ channels. J Biol Chem. 1999;274:16973–16978. 6. Loffing J, Zecevic M, Feraille E, Kaissling B, Asher C, Rossier BC, Firestone GL, Pearce D, Verrey F. Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. Am J Physiol Renal Physiol. 2001;280:F675–F682. 7. Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci U S A. 1999;96: 2514–2519. 8. Bohmer C, Wagner CA, Beck S, Moschen V, Melzig J, Werner A, Lin JT, Lang F, Wehner F. The shrinkage-activated Na+ conductance of rat hepatocytes and its possible correlation to rENaC. Cell Physiol Biochem. 2000;10: 187–194. 9. Wagner CA, Ott M, Klingel K, Beck S, Melzig J, Friedrich B, Wild KN, Broer S, Moschen I, Albers A, Waldegger S, Tummler B, Egan ME, Geibel JP, Kandolf R, Lang F. Effects of the serine/threonine kinase SGK1 on the epithelial Na+ channel (ENaC) and CFTR: implications for cystic fibrosis. Cell Physiol Biochem. 2001;11:209–218. |
The source is not mentioned. |
|
| [10.] Dsa/Fragment 034 00 - Diskussion Bearbeitet: 18. August 2016, 20:44 WiseWoman Erstellt: 17. May 2015, 23:19 (Hindemith) | Debonneville et al 2001, Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 34, Zeilen: figure |
Quelle: Debonneville et al 2001 Seite(n): 7053, Zeilen: figure |
|---|---|
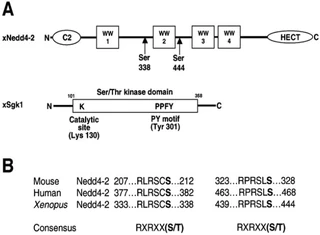
Figure nr. 7 - Schematic view of Nedd4-2 and Sgk1. (A) Scheme of Xenopus Nedd4-2 with the consensus phosphorylation sites and Xenopus Sgk1 with the indication of the catalytic domain, the catalytically essential Lys130 and the PY motif. (B) Conserved consensus phosphorylation sites in mouse, human and Xenopus Nedd4-2. |
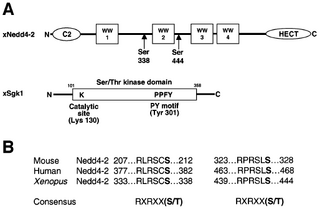
Fig. 1. Schematic view of Nedd4-2 and Sgk1. (A) Scheme of Xenopus Nedd4-2 with the consensus phosphorylation sites and Xenopus Sgk1 with the indication of the catalytic domain, the catalytically essential Lys130 and the PY motif. (B) Conserved consensus phosphorylation sites in mouse, human and Xenopus Nedd4-2. |
The source is mentioned on the previous page, but without indication that the figure is taken from it. |
|
| [11.] Dsa/Fragment 038 00 - Diskussion Bearbeitet: 18. August 2016, 20:38 WiseWoman Erstellt: 17. May 2015, 23:04 (Hindemith) | Amorim et al 2004, Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 38, Zeilen: figure |
Quelle: Amorim et al 2004 Seite(n): 702, Zeilen: figure |
|---|---|
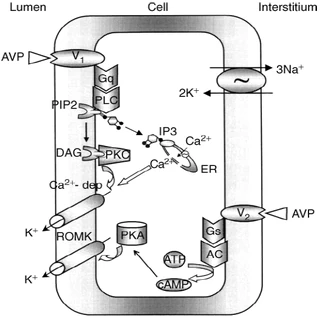
Figure nr. 8 - Schematic drawing of cell signalling mechanisms of luminal (V1 receptors) and basolateral (V2 receptors) action of arginine vasopressin (AVP). The role of V1 receptors and their signalling are derived from the present studies. The role of V2 receptors in ROMK channel stimulation is based on the studies of Cassola, Giebisch and Wang. |
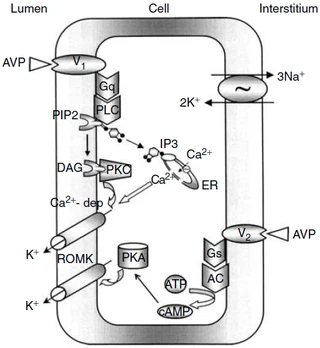
Fig. 7. Schematic drawing of cell signaling mechanisms of luminal (V1 receptors) and basolateral (V2 receptors) action of arginine vasopressin (AVP). The role of V1 receptors and their signaling are derived from the present studies. The role of V2 receptors in ROMK channel stimulation is based on the studies of Cassola, Giebisch, and Wang [6]. 6. CASSOLA AC, GIEBISCH G, WANG W: Vasopressin increases density of apical low-conductance K+ channels in rat CCD. Am J Physiol Renal Fluid Electrolyte Physiol 264:F502–F509, 1993 |
The source is not mentioned. Note that no paper of Cassola et al. is listed in the bibliography. |
|
| [12.] Dsa/Fragment 045 06 - Diskussion Bearbeitet: 9. August 2016, 21:02 WiseWoman Erstellt: 17. May 2015, 21:24 (Hindemith) | Dsa, Fragment, Gesichtet, KomplettPlagiat, Lang et al 2006, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 45, Zeilen: 6-12 |
Quelle: Lang et al 2006 Seite(n): 1156, Zeilen: r.col: 16ff |
|---|---|
| As shown in Xenopus oocytes, SGK1 and SGK3 activate the renal epithelial Ca2+ channel TRPV5 by enhancing channel abundance in the plasma membrane, an effect again requiring cooperation with NHERF2 (Embark HM. et al., (2004) Cell Physiol Biochem; Palmada M. et al., (2005) Cell Physiol Biochem). The TRPV5 C-tail interacts in a Ca2+- independent manner with NHERF2. Deletion of the second, but not the first, PDZ domain in NHERF2 abrogates the stimulating effect of SGK1 on TRPV5 protein abundance (Palmada M. et al., (2005) Cell Physiol Biochem). | As shown in Xenopus oocytes, SGK1 and SGK3 activate the renal epithelial Ca2+ channel TRPV5 by enhancing channel abundance in the plasma membrane, an effect again requiring cooperation with NHERF2 (101, 246). The TRPV5 C-tail interacts in a Ca2+-independent manner with NHERF2. Deletion of the second, but not the first, PDZ domain in NHERF2 abrogates the stimulating effect of SGK1 on TRPV5 protein abundance (246).
101. Embark HM, Setiawan I, Poppendieck S, van de Graaf SF, Boehmer C, Palmada M, Wieder T, Gerstberger R, Cohen P, Yun CC, Bindels RJ, and Lang F. Regulation of the epithelial Ca2+ channel TRPV5 by the NHE regulating factor NHERF2 and the serum and glucocorticoid inducible kinase isoforms SGK1 and SGK3 expressed in Xenopus oocytes. Cell Physiol Biochem 14: 203–212, 2004. 246. Palmada M, Poppendieck S, Embark HM, van de Graaf SF, Boehmer C, Bindels RJ, and Lang F. Requirement of PDZ domains for the stimulation of the epithelial Ca2+ channel TRPV5 by the NHE regulating factor NHERF2 and the serum and glucocorticoid inducible kinase SGK1. Cell Physiol Biochem 15: 175–182, 2005. |
The source is not given. |
|
| [13.] Dsa/Fragment 033 00 - Diskussion Bearbeitet: 9. August 2016, 20:57 WiseWoman Erstellt: 17. May 2015, 21:30 (Hindemith) | Dsa, Fragment, Gesichtet, KomplettPlagiat, Lang et al 2006, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 33, Zeilen: figure+caption |
Quelle: Lang et al 2006 Seite(n): 1154, Zeilen: figure |
|---|---|
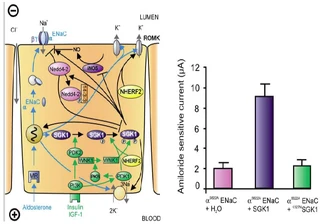
Figure nr. 6 - Left: model for the Serum- and Glucocorticoid-inducible Kinase-1 (SGK1)-dependent regulation of Na+ reabsorption and K+ secretion in the aldosterone-sensitive distal nephron. Aldosterone binds to mineralocorticoid receptors (MR) and stimulates the expression of SGK1, α-epithelial Na+ channel (αENaC), renal outer medullary K+ channel (ROMK), and the Na+-K+-ATPase. αENaC associates with constitutive β- and γ-subunits to form fully active ENaC. SGK1 can be phosphorylated on 422Ser by insulin or insulin-like growth factor I (IGF-I) through a signaling cascade involving phosphatidylinositol 3-kinase (PI3K) and an unknown kinase (PDK2?/hydrophobic motif kinase). Phosphorylated 422Ser allows binding of PDK1 and/or NHERF2 with subsequent phosphorylation of SGK1 at 256Thr. PDK1 might activate SGK1 indirectly through phosphorylation of WNK1 kinase. The mechanism of SGK1 activation by WNK1 is yet unknown but does not require SGK1 phosphorylation. Activated SGK1 increases Na+ reabsorption in part by phosphorylation of the ubiquitin ligase Nedd4–2, allowing binding of the chaperone 14–3-3 to phosphorylated 444Ser. This interaction prevents Nedd4–2-mediated ubiquitination of the ENaC-PY motif and thus internalization and degradation of ENaC. SGK1 further stimulates ENaC by upregulation of transcription, by direct phosphorylation of the channel protein and by inhibition of the inducible nitric oxide synthase (iNOS). In addition to its effect on ENaC, SGK1 stimulates the Na+-K+-ATPase and K+ channels including ROMK. Right: arithmetic means ± SE of ENaC-induced currents in Xenopus oocytes coexpression experiments showing that coexpression of wild-type SGK1 but not of the inactive mutant K127NSGK1 leads to stimulation of an ENaC mutant lacking the SGK1 phosphorylation consensus sequence (S622AENaC). |
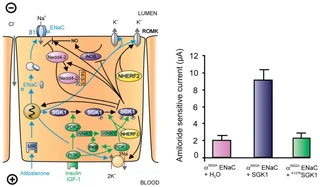
FIG. 1. Left: model for the Serum- and Glucocorticoid-inducible Kinase-1 (SGK1)-dependent regulation of Na+ reabsorption and K+ secretion in the aldosterone-sensitive distal nephron. Aldosterone binds to mineralocorticoid receptors (MR) and stimulates the expression of SGK1, α-epithelial Na+ channel (αENaC), renal outer medullary K+ channel (ROMK), and the Na+-K+-ATPase. αENaC associates with constitutive β- and γ-subunits to form fully active ENaC. SGK1 can be phosphorylated on 422Ser by insulin or insulin-like growth factor I (IGF-I) through a signaling cascade involving phosphatidylinositol 3-kinase (PI 3K) and an unknown kinase (PDK2?/hydrophobic motif kinase). Phosphorylated 422Ser allows binding of PDK1 and/or NHERF2 with subsequent phosphorylation of SGK1 at 256Thr. PDK1 might activate SGK1 indirectly through phosphorylation of WNK1 kinase. Phosphorylated 422Ser allows binding of PDK1 and/or NHERF2 with subsequent phosphorylation of SGK1 at 256Thr. PDK1 might activate SGK1 indirectly through phosphorylation of WNK1 kinase. The mechanism of SGK1 activation by WNK1 is yet unknown but does not require SGK1 phosphorylation. Activated SGK1 increases Na+ reabsorption in part by phosphorylation of the ubiquitin ligase Nedd4–2, allowing binding of the chaperone 14–3-3 to phosphorylated 444Ser. This interaction prevents Nedd4–2-mediated ubiquitination of the ENaC-PY motif and thus internalization and degradation of ENaC. SGK1 further stimulates ENaC by upregulation of transcription, by direct phosphorylation of the channel protein, and by inhibition of the inducible nitric oxide synthase (iNOS). In addition to its effect on ENaC, SGK1 stimulates the Na+-K+-ATPase and K+ channels including ROMK. Right: arithmetic means ± SE of ENaC-induced currents in Xenopus oocyte coexpression experiments showing that coexpression of wild-type SGK1 but not of the inactive mutant K127NSGK1 leads to stimulation of an ENaC mutant lacking the SGK1 phosphorylation consensus sequence (S622AENaC). |
The source is not given. |
|
| [14.] Dsa/Fragment 031 01 - Diskussion Bearbeitet: 9. August 2016, 20:47 WiseWoman Erstellt: 17. May 2015, 21:38 (Hindemith) | Dsa, Fragment, Gesichtet, KomplettPlagiat, Lang et al 2006, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 31, Zeilen: 1-32 |
Quelle: Lang et al 2006 Seite(n): 1151: 1152, 1155, Zeilen: 1151: abstract; 1152: r.col: 5ff; 1155: l.col: 3ff |
|---|---|
| 1.7. Degradation of SGKs
SGK1 is rapidly degraded, with a half-life of 30 min (Brickley DR. et al., (2002) J Biol Chem). Ubiquitination of SGK1 labels the kinase for degradation by the proteasome (Brickley DR. et al., (2002) J Biol Chem). SGK1 degradation may be mediated by the ubiquitin ligase Nedd4–2 (neuronal precursor cells expressed developmentally downregulated) (Zhou R.et al., (2005) Biol Chem). Nedd4–2 contains a series of tryptophan-rich sequences (WW motifs) that interact with a proline-tyrosine PY motif present in its target proteins. SGK1 bears such a PY motif. Overexpression of Nedd4–2 decreases steady state levels of SGK1 in a dose-dependent manner by increasing SGK1 ubiquitination (presumably within the first 60 NH2-terminal amino acids) and subsequent degradation in the 26S proteasome. Conversely, silencing of Nedd4–2 by RNA interference, or loss of the NH2-terminal amino acids, abrogates the ubiquitination and thus increases the half-life of SGK1. The effect of Nedd4–2 apparently requires phosphorylation of the ubiquitin ligase by SGK1, as SGK1 degradation is reduced by a phosphorylation site-deficient Nedd4–2 mutant (Nedd4–2S/T-A) or by SGK1 inhibition. Accordingly, active SGK1 favors its own degradation, thus contributing to the limitation of its action (Zhou R. et al., (2005) J Biol Chem). 1.8. Influence of SGK on renal function SGKs activate ion channels (e.g. ENaC, TRPV5, ROMK, Kv1.3, KCNE1/KCNQ1, GluR1, GluR6), carriers (e.g. NHE3, GLUT1, SGLT1, EAAT1–5), and the Na+-K+-ATPase. They regulate the activity of enzymes (e.g. glycogen synthase kinase-3, ubiquitin ligase Nedd4–2, phosphomannose mutase-2) and transcription factors (e.g. forkhead transcription factor FKHRL1). The functional significance of SGK1, SGK2, and SGK3 is still far from understood. Notably, all three kinases are potent regulators of ion channel activity, transport, and transcription (Bhargava A. and Pearce D., (2004) Trends Endocrinol Metab; Fillon S. et al., (2001) Comp Biochemi Physiol Mol Integr Physiol). Functional analysis of gene-targeted mice lacking SGK1 (Wulff P. et al., (2002) J Clin Invest) and SGK3 (McCormick JA. et al., (2004) Moll Biol Cell) provided insight into the functional significance of SGK1- and SGK3-dependent regulation of physiological functions. Interestingly, neither knockout of SGK1 or SGK3, nor knockout of both SGK1 and SGK3 leads to a severe phenotype, suggesting that neither SGK1 nor SGK3 is required for survival. Closer inspection of the renal physiology of those mice discloses, however, multiple physiological deficits pointing to the broad functional role of these kinases. |
SGKs activate ion channels (e.g., ENaC, TRPV5, ROMK, Kv1.3, KCNE1/KCNQ1, GluR1, GluR6), carriers (e.g., NHE3, GLUT1, SGLT1, EAAT1–5), and the Na+-K+-ATPase. They regulate the activity of enzymes (e.g., glycogen synthase kinase-3, ubiquitin ligase Nedd4–2, phosphomannose mutase-2) and transcription factors (e.g., forkhead transcription factor FKHRL1, β-catenin, nuclear factor κB).
The functional significance of SGK1, SGK2, and SGK3 is still far from understood. Notably, all three kinases are potent regulators of ion channel activity, transport, and transcription (30, 111, 183, 186, 250, 305, 331, 378). Functional analysis of gene-targeted mice lacking SGK1 (368) and SGK3 (214) provided insight into the functional significance of SGK1- and SGK3-dependent regulation of physiological functions. Interestingly, neither knockout of SGK1 (368) or SGK3 (214), nor knockout of both SGK1 and SGK3 (133) leads to a severe phenotype, suggesting that neither SGK1 nor SGK3 is required for survival. Closer inspection of the physiology of those mice discloses, however, multiple physiological deficits pointing to the broad functional role of these kinases. [page 1155] C. Degradation of SGKs SGK1 is rapidly degraded, with a half-life of 30 min (50). Ubiquitination of SGK1 labels the kinase for degradation by the proteasome (50). SGK1 degradation may be mediated by the ubiquitin ligase Nedd4–2 (neuronal precursor cells expressed developmentally downregulated) (386). Nedd4–2 contains a series of tryptophan-rich sequences (WW motifs) that interact with a proline-tyrosine PY motif present in its target proteins. SGK1 bears such a PY motif. Overexpression of Nedd4–2 decreases steady-state levels of SGK1 in a dose-dependent manner by increasing SGK1 ubiquitination (presumably within the first 60 NH2-terminal amino acids) and subsequent degradation in the 26S proteasome. Conversely, silencing of Nedd4–2 by RNA interference, or loss of the NH2-terminal amino acids, abrogates the ubiquitination and thus increases the half-life of SGK1. The effect of Nedd4–2 apparently requires phosphorylation of the ubiquitin ligase by SGK1, as SGK1 degradation is reduced by a phosphorylation site-deficient Nedd4–2 mutant (Nedd4–2S/T-A) or by SGK1 inhibition (Fig. 1). Accordingly, active SGK1 favors its own degradation, thus contributing to the limitation of its action (386). 30. Bhargava A and Pearce D. Mechanisms of mineralocorticoid action: determinants of receptor specificity and actions of regulated gene products. Trends Endocrinol Metab 15: 147–153, 2004. 50. Brickley DR, Mikosz CA, Hagan CR, and Conzen SD. Ubiquitin modification of serum and glucocorticoid-induced protein kinase-1 (SGK-1). J Biol Chem 277: 43064–43070, 2002. 111. Fillon S, Warntges S, Matskevitch J, Moschen I, Setiawan I, Gamper N, Feng YX, Stegen C, Friedrich B, Waldegger S, Broer S, Wagner CA, Huber SM, Klingel K, Vereninov A, and Lang F. Serum- and glucocorticoid-dependent kinase, cell volume, and the regulation of epithelial transport. Comp Biochem Physiol A Mol Integr Physiol 130: 367–376, 2001. 133. Grahammer F, Henke G, Sandu C, Rexhepaj R, Hussain A, Friedrich B, Risler T, Just L, Skutella T, Wulff P, Kuhl D, and Lang F. Intestinal function of gene targeted mice lacking the serum and glucocorticoid inducible kinase SGK1. Am J Physiol Gastrointest Liver Physiol 290: G1114–G1123, 2006. 183. Lang F, Henke G, Embark HM, Waldegger S, Palmada M, Bohmer C, and Vallon V. Regulation of channels by the serum and glucocorticoid-inducible kinase: implications for transport, excitability and cell proliferation. Cell Physiol Biochem 13: 41–50, 2003. 186. Lang F, Vallon V, Grahammer F, Palmada M, and Bohmer C. Transport regulation by the serum- and glucocorticoid-inducible kinase SGK1. Biochem Soc Trans 33: 213–215, 2005. 214. McCormick JA, Feng Y, Dawson K, Behne MJ, Yu B, Wang J, Wyatt AW, Henke G, Grahammer F, Mauro TM, Lang F, and Pearce D. Targeted disruption of the protein kinase SGK3/CISK impairs postnatal hair follicle development. Mol Biol Cell 15: 4278– 4288, 2004. 250. Pearce D. SGK1 regulation of epithelial sodium transport. Cell Physiol Biochem 13: 13–20, 2003. 305. Stockand JD. New ideas about aldosterone signaling in epithelia. Am J Physiol Renal Physiol 282: F559–F576, 2002. 331. Verrey F, Loffing J, Zecevic M, Heitzmann D, and Staub O. SGK1: aldosterone-induced relay of Na+ transport regulation in distal kidney nephron cells. Cell Physiol Biochem 13: 21–28, 2003. 368. Wulff P, Vallon V, Huang DY, Volkl H, Yu F, Richter K, Jansen M, Schlunz M, Klingel K, Loffing J, Kauselmann G, Bosl MR, Lang F, and Kuhl D. Impaired renal Na+ retention in the sgk1-knockout mouse. J Clin Invest 110: 1263–1268, 2002. 378. Yun CC. Concerted roles of SGK1 and the Na+/H+ exchanger regulatory factor 2 (NHERF2) in regulation of NHE3. Cell Physiol Biochem 13: 029–040, 2003. 386. Zhou R and Snyder PM. Nedd4–2 phosphorylation induces serum and glucocorticoid-regulated kinase (SGK) ubiquitination and degradation. J Biol Chem 280: 4518–4523, 2005. |
The source is not given. |
|
| [15.] Dsa/Fragment 030 01 - Diskussion Bearbeitet: 9. August 2016, 20:33 WiseWoman Erstellt: 17. May 2015, 21:57 (Hindemith) | Dsa, Fragment, Gesichtet, KomplettPlagiat, Lang et al 2006, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 30, Zeilen: 1ff (entire page) |
Quelle: Lang et al 2006 Seite(n): 1153, 1154, Zeilen: 1153: r.col: 47ff; 1154: l.col: 1ff |
|---|---|
| [Recent evidence] suggested a role of WNK1 in the activation of SGK1 by IGF-I (Xu BE. et al., (2005) J Biol Chem). According to this evidence, IGF-I induces SGK1 activity by stimulating WNK1 phosphorylation at 58Thr, a site that is phosphorylated by protein kinase B (PKB/Akt). The PI3-kinase-dependent step in the activation of SGK1 by IGF-I was thus suggested to be the PDK1-dependent activation of PKB/Akt and the subsequent phosphorylation of WNK1 at 58Thr (Xu BE. et al., (2005) J Biol Chem). Neither the catalytic activity nor the kinase domain but the NH2 [sic] -terminal 220 residues of WNK1 are required for activation of SGK1 (Xu BE. et al., (2005) J Biol Chem). WNK1 binds SGK1 directly but does not phosphorylate it, suggesting that WNK1 serves as a scaffold protein to assemble other molecules required for maximal SGK1 activation. Its phosphorylation at 58Thr by PKB-Akt may induce binding of accessory proteins or a conformational change in SGK1 that stimulates the kinase. However, further experimental evidence is needed to elucidate how WNK1 phosphorylation promotes SGK1 activation. SGK2 and SGK3 may similarly be activated by PDK1 and PDK2/H-motif kinase. The equivalent phosphorylation sites for SGK2 and SGK3 are predicted to be at 193Thr-356Ser and 253Thr-419Ser, respectively, but this requires further investigation. The kinases are also regulated by WNK1, although to a lesser extent than SGK1 (Xu BE. et al., (2005) J Biol Chem).
Replacement of the serine at position 422 by aspartate, in the human SGK, leads to the constitutively active S422DSGK1 (Kobayashi T. et al., (1999) Biochem J), whereas replacement of lysine at position 127, within the ATP-binding region required for enzymatic activity, with asparagine leads to the inactive K127NSGK1 (Kobayashi T. et al., (1999) Biochem J). Analogous mutations in the human SGK2 and SGK3 lead to the constitutively active S356DSGK2 and S419DSGK3 and the constitutively inactive K64NSGK2 and K191NSGK3. In part through the PI3-kinase pathway, SGK1 is activated by insulin (Kobayashi T. et al., (1999) Biochem J), IGF-I (Hayashi M. et al., (2001) J Biol Chem; Kobayashi T. et al., (1999) Biochem J), hepatic growth factor (HGF) (Shelly C. et al., (2002) J Cell Sci) and follicle stimulating hormone (FSH) (Richards JS. et al., (2002) Mol Endocrinol). SGK1 can be activated by bone marrow kinase/extracellular signal-regulated kinase 5 (BK/ERK5) or by p38α. The kinases do not phosphorylate SGK1 at 256Thr but at 78Ser, which is outside the catalytic domain (Hayashi M. et al., (2001) J Biol Chem; Meng F. et al., (2005) Am J Physiol Cell Physiol). How this phosphorylation activates SGK1 is not known. SGK1 can also be activated by an increase of cytosolic Ca2+ activity, an effect presumably mediated by calmodulin - dependent protein kinase kinase (CaMKK) (Imai S. et al., (2003) Life Sci). Moreover, the small G protein Rac1 activates SGK1 via a PI3-kinaseindependent pathway (Shelly C. et al., (2002) J Cell Sci). Additional activators of SGK1 include neuronal depolarization (Kumari S. et al., (2001) Brain Res), cAMP (Kumari S. et al., (2001) Brain Res; Perrotti N. et al., (2001) J Biol Chem; Thomas CP. et al., (2004) Am J Physiol Lung Cell Mol Physiol), lithium (Kumari S. et al., (2001) Brain Res), oxidation (Kobayashi T and Cohen P., (1999) Biochem J; Prasad N. et al., (2000) Biochemistry) and adhesion to fibronectin (Shelly C. et al., (2002) J Cell Sci). Similar to SGK1, SGK2 and SGK3 are activated by oxidation, insulin, and IGF-I through a signaling cascade. |
Recent evidence suggested a role of WNK1 in the activation of SGK1 by IGF-I (371). According to this evidence, IGF-I induces SGK1 activity by stimulating WNK1 phosphorylation at 58Thr, a site that is phosphorylated by protein kinase B (PKB/Akt). The PI 3-kinasedependent step in the activation of SGK1 by IGF-I was
[page 1154] thus suggested to be the PDK1-dependent activation of PKB/Akt and the subsequent phosphorylation of WNK1 at 58Thr (371). Neither the catalytic activity nor the kinase domain but the NH2-terminal 220 residues of WNK1 are required for activation of SGK1 (371). WNK1 binds SGK1 directly but does not phosphorylate it, suggesting that WNK1 serves as a scaffold protein to assemble other molecules required for maximal SGK1 activation. Its phosphorylation at 58Thr by PKB/Akt may induce binding of accessory proteins or a conformational change in SGK1 that stimulates the kinase. However, further experimental evidence is needed to elucidate how WNK1 phosphorylation promotes SGK1 activation. SGK2 and SGK3 may similarly be activated by PDK1 and PDK2/H-motif kinase. The equivalent phosphorylation sites for SGK2 and SGK3 are predicted to be at 193Thr/356Ser and 253Thr/419Ser, respectively, but this requires further investigation. The kinases are also regulated by WNK1, although to a lesser extent than SGK1 (371). Replacement of the serine at position 422 by aspartate in the human SGK1 leads to the constitutively active S422DSGK1 (172), whereas replacement of lysine at position 127, within the ATP-binding region required for enzymatic activity, with asparagine leads to the inactive K127NSGK1 (172). Analogous mutations in the human SGK2 and SGK3 lead to the constitutively active S356DSGK2 and S419DSGK3 and the constitutively inactive K64NSGK2 and K191NSGK3 (41). In part through the PI 3-kinase pathway, SGK1 is activated by insulin (171, 254), IGF-I (137, 171, 179), hepatic growth factor (HGF) (287), and follicle stimulating hormone (FSH) (265). SGK1 can be activated by bone marrow kinase/extracellular signal-regulated kinase 5 (BK/ERK5) or by p38α. The kinases do not phosphorylate SGK1 at 256Thr but at 78Ser, which is outside the catalytic domain (137, 216). How this phosphorylation activates SGK1 is not known. SGK1 can also be activated by an increase of cytosolic Ca2+ activity, an effect presumably mediated by calmodulin-dependent protein kinase kinase (CaMKK) (158). Moreover, the small G protein Rac1 activates SGK1 via a PI 3-kinase-independent pathway (287). Additional activators of SGK1 include neuronal depolarization (179), cAMP (179, 254, 315), lithium (179), oxidation (171, 256), and adhesion to fibronectin (287). Similar to SGK1, SGK2 and SGK3 are activated by oxidation, insulin, and IGF-I through a signaling cascade [page 1155] involving PI 3-kinase as well as PDK1 and PDK2/H-motif kinase (171, 335). 137. Hayashi M, Tapping RI, Chao TH, Lo JF, King CC, Yang Y, and Lee JD. BMK1 mediates growth factor-induced cell proliferation through direct cellular activation of serum and glucocorticoidinducible kinase. J Biol Chem 276: 8631–8634, 2001. 158. Imai S, Okayama N, Shimizu M, and Itoh M. Increased intracellular calcium activates serum and glucocorticoid-inducible kinase 1 (SGK1) through a calmodulin-calcium calmodulin dependent kinase kinase pathway in Chinese hamster ovary cells. Life Sci 72: 2199–2209, 2003. 171. Kobayashi T and Cohen P. Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphati-dylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem J 339: 319–328, 1999. 172. Kobayashi T, Deak M, Morrice N, and Cohen P. Characterization of the structure and regulation of two novel isoforms of serum and glucocorticoid-induced protein kinase. Biochem J 344: 189–197, 1999. 179. Kumari S, Liu X, Nguyen T, Zhang X, and D’Mello SR. Distinct phosphorylation patterns underlie Akt activation by different survival factors in neurons. Brain Res 96: 157–162, 2001. 216. Meng F, Yamagiwa Y, Taffetani S, Han J, and Patel T. IL-6 activates serum and glucocorticoid kinase via p38alpha mitogenactivated protein kinase pathway. Am J Physiol Cell Physiol 289: C971–C981, 2005. 254. Perrotti N, He RA, Phillips SA, Haft CR, and Taylor SI. Activation of serum- and glucocorticoid-induced protein kinase (Sgk) by cyclic AMP and insulin. J Biol Chem 276: 9406–9412, 2001. 256. Prasad N, Topping RS, Zhou D, and Decker SJ. Oxidative stress and vanadate induce tyrosine phosphorylation of phosphoinositide-dependent kinase 1 (PDK1). Biochemistry 39: 6929–6935, 2000. 265. Richards JS, Sharma SC, Falender AE, and Lo YH. Expression of FKHR, FKHRL1, and AFX genes in the rodent ovary: evidence for regulation by IGF-I, estrogen, and the gonadotropins. Mol Endocrinol 16: 580–599, 2002. 287. Shelly C and Herrera R. Activation of SGK1 by HGF, Rac1 and integrin-mediated cell adhesion in MDCK cells: PI-3K-dependent and -independent pathways. J Cell Sci 115: 1985–1993, 2002. 315. Thomas CP, Campbell JR, Wright PJ, and Husted RF. cAMPstimulated Na+ transport in H441 distal lung epithelial cells: role of PKA, phosphatidylinositol 3-kinase, and sgk1. Am J Physiol Lung Cell Mol Physiol 287: L843–L851, 2004. 335. Virbasius JV, Song X, Pomerleau DP, Zhan Y, Zhou GW, and Czech MP. Activation of the Akt-related cytokine-independent survival kinase requires interaction of its phox domain with endosomal phosphatidylinositol 3-phosphate. Proc Natl Acad Sci USA 98: 12908–12913, 2001. 371. Xu BE, Stippec S, Lazrak A, Huang CL, and Cobb MH. WNK1 activates SGK1 by a phosphatidylinositol 3-kinase-dependent and non-catalytic mechanism. J Biol Chem 280: 34218–34223, 2005. |
The source is not mentioned. Note that the last sentence ends abruptly together with the page break in the source. |
|
| [16.] Dsa/Fragment 029 14 - Diskussion Bearbeitet: 9. August 2016, 20:11 WiseWoman Erstellt: 17. May 2015, 22:07 (Hindemith) | Dsa, Fragment, Gesichtet, KomplettPlagiat, Lang et al 2006, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 29, Zeilen: 14-41 |
Quelle: Lang et al 2006 Seite(n): 1153, Zeilen: r.col: 13ff |
|---|---|
| 1.6. Regulation of SGK kinase activity
To become functional, the SGK protein kinases require activation by phosphorylation, which is accomplished through a signaling cascade involving PI 3-kinase, the 3- phosphoinositide (PIP3)-dependent kinase PDK1, and a yet unidentified but also PIP3- dependent kinase that has been referred to as PDK2 or “hydrophobic motif” (H-motif) kinase (Collins BJ. et al., (2003) EMBO J; Frodin M. et al., (2002) EMBO J; Kobayashi T. et al., (1999) Biochem J; Mora A. et al., (2004) Semin Cell Dev Biol; Nilsen T. et al., (2004) J Biol Chem; Park J. et al., (1999) EMBO J). PIP3 is degraded by the phosphatase and tensin homolog PTEN (Lian Z. et al., (2005) Oncogene; Oudit GY. et al., (2004) J Mol Cell Cardiol; Sulis ML. et al., (2003) Trends Cell Biol), which thus disrupts PI 3-kinasedependent activation of the SGKs. Maximal stimulation of SGK1 activity requires the PDK1-dependent phosphorylation at 256Thr within the activation loop (T-loop) and phosphorylation at 422Ser in the hydrophobic motif at its COOH terminus by PDK2/H-motif kinase (Kobayashi T. et al., (1999) Biochem J; Park J. et al., (1999) EMBO J). The PDK1-mediated SGK1 phosphorylation is facilitated when 422Ser is already phosphorylated. Phosphorylation of SGK1 at 422Ser promotes SGK1 binding to the PDK1 interacting fragment (PIF)-binding pocket and phosphorylation at 256Thr by PDK1 (Biondi RM. et al., (2001) EMBO J). An alternate mechanism of SGK1 activation by PDK1 involves the scaffold protein Na+-H+ exchanger regulating factor 2 (NHERF2). NHERF2 mediates the assembly of SGK1 and PDK1 via its PDZ domains and PIF consensus sequence (Chun J. et al., (2003) J Biochem Tokyo). NHERF2 interacts with the PDZ binding motif of SGK1 through its first PDZ domain and with PIF-binding pocket of PDK1 through its PIF tail. The formation of the ternary complex facilitates the phosphorylation of SGK1 on 256Thr in its T-loop by PDK1 (Chun J. et al., (2003) J Biochem Tokyo). Most recent evidence suggests that the activation of SGK1 by PDK1 may indirectly involve the serine/threonine kinase WNK1 (with no lysine kinase 1) (Xu BE. et al., (2005) Proc Natl Acad Sci Usa). It is well established that insulin-like growth factor I (IGF-I) enhances SGK1 activity in a PI3-kinase-dependent manner via PDK1. |
B. Regulation of SGK Kinase Activity
To become functional, the SGK protein kinases require activation by phosphorylation, which is accomplished through a signaling cascade involving PI 3-kinase, the 3-phosphoinositide (PIP3)-dependent kinase PDK1, and a yet unidentified but also PIP3-dependent kinase that has been referred to as PDK2 or “hydrophobic motif” (H-motif) kinase (5, 31, 75, 119, 171, 224, 235, 249, 369). PIP3 is degraded by the phosphatase and tensin homolog PTEN (202, 240, 309), which thus disrupts PI 3-kinasedependent activation of the SGKs. Maximal stimulation of SGK1 activity requires the PDK1-dependent phosphorylation at 256Thr within the activation loop (T-loop) and phosphorylation at 422Ser in the hydrophobic motif at its COOH terminus by PDK2/H-motif kinase (171, 172, 249). The PDK1-mediated SGK1 phosphorylation is facilitated when 422Ser is already phosphorylated (Fig. 1). Phosphorylation of SGK1 at 422Ser promotes SGK1 binding to the PDK1 interacting fragment (PIF)-binding pocket and phosphorylation at 256Thr by PDK1 (31). An alternate mechanism of SGK1 activation by PDK1 involves the scaffold protein Na+/H+ exchanger regulating factor 2 (NHERF2). NHERF2 mediates the assembly of SGK1 and PDK1 via its PDZ domains and PIF consensus sequence (70). NHERF2 interacts with the PDZ binding motif of SGK1 through its first PDZ domain and with PIF-binding pocket of PDK1 through its PIF tail. The formation of the ternary complex facilitates the phosphorylation of SGK1 on 256Thr in its T-loop by PDK1 (70). Most recent evidence suggests that the activation of SGK1 by PDK1 may indirectly involve the serine/threonine kinase WNK1 (with no lysine kinase 1) (370). It is well established that insulin-like growth factor I (IGF-I) enhances SGK1 activity in a PI 3-kinase-dependent manner via PDK1. 5. Alessi DR, Deak M, Casamayor A, Caudwell FB, Morrice N, Norman DG, Gaffney P, Reese CB, MacDougall CN, Harbison D, Ashworth A, and Bownes M. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol 7: 776–789, 1997. 31. Biondi RM, Kieloch A, Currie RA, Deak M, and Alessi DR. The PIF-binding pocket in PDK1 is essential for activation of S6K and SGK, but not PKB. EMBO J 20: 4380–4390, 2001. 70. Chun J, Kwon T, Lee E, Suh PG, Choi EJ, and Sun KS. The Na(+)/H(+) exchanger regulatory factor 2 mediates phosphorylation of serum- and glucocorticoid-induced protein kinase 1 by 3-phosphoinositide-dependent protein kinase 1. Biochem Biophys Res Commun 298: 207–215, 2002. 75. Collins BJ, Deak M, Arthur JS, Armit LJ, and Alessi DR. In vivo role of the PIF-binding docking site of PDK1 defined by knock-in mutation. EMBO J 22: 4202–4211, 2003. 119. Frodin M, Antal TL, Dummler BA, Jensen CJ, Deak M, Gammeltoft S, and Biondi RM.'#' A phosphoserine/threonine-binding pocket in AGC kinases and PDK1 mediates activation by hydrophobic motif phosphorylation. EMBO J 21: 5396–5407, 2002. 171. Kobayashi T and Cohen P. Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphati-dylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem J 339: 319–328, 1999. 172. Kobayashi T, Deak M, Morrice N, and Cohen P. Characterization of the structure and regulation of two novel isoforms of serumand glucocorticoid-induced protein kinase. Biochem J 344: 189–197, 1999. 202. Lian Z and Di Cristofano A. Class reunion: PTEN joins the nuclear crew. Oncogene 24: 7394–7400, 2005. 224. Mora A, Komander D, van Aalten DM, and Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol 15: 161–170, 2004. 235. Nilsen T, Slagsvold T, Skjerpen CS, Brech A, Stenmark H, and Olsnes S. Peroxisomal targeting as a tool for assaying protein-protein interactions in the living cell: cytokine-independent survival kinase (CISK) binds PDK-1 in vivo in a phosphorylationdependent manner. J Biol Chem 279: 4794–4801, 2004. 240. Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, and Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol 37: 449–471, 2004. 249. Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, and Hemmings BA. Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. EMBO J 18: 3024–3033, 1999. 309. Sulis ML and Parsons R. PTEN: from pathology to biology. Trends Cell Biol 13: 478–483, 2003. 369. Xing Y, Liu D, Zhang R, Joachimiak A, Songyang Z, and Xu W. Structural basis of membrane targeting by the Phox homology domain of cytokine-independent survival kinase (CISK-PX). J Biol Chem 279: 30662–30669, 2004. 370. Xu BE, Stippec S, Chu PY, Lazrak A, Li XJ, Lee BH, English JM, Ortega B, Huang CL, and Cobb MH. WNK1 activates SGK1 to regulate the epithelial sodium channel. Proc Natl Acad Sci USA 102: 10315–10320, 2005. |
The source is not mentioned here. |
|
| [17.] Dsa/Fragment 026 01 - Diskussion Bearbeitet: 8. August 2016, 11:34 WiseWoman Erstellt: 18. May 2015, 09:37 (Hindemith) | Dsa, Fragment, Gesichtet, KomplettPlagiat, Lang et al 2006, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 26, Zeilen: 1-9, 14-18 |
Quelle: Lang et al 2006 Seite(n): 1152, 1160, Zeilen: 1152: 3ff, l.col: last paragraph; 1160: figure |
|---|---|
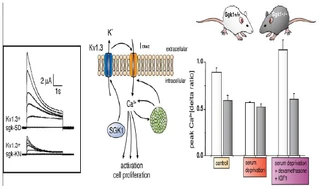
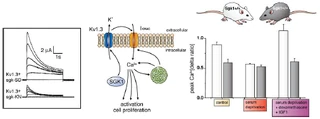
Figure nr. 4 - Middle: putative role of SGK1 in the regulation of K+ and Ca2+ channels required for stimulation of cell proliferation. Mitogenic factors stimulate the Ca2+ release-activated Ca2+ channel ICRAC. Ca2+ entry through this channel is highly sensitive to cell membrane potential, which is maintained by K+ channels. The stimulation of the voltage-sensitive K+ channel Kv1.3 by SGK1 may serve to maintain the cell membrane polarization and thus sustain oscillating Ca2+ entry through ICRAC. Left: current generated by depolarization of HEK cells overexpressing constitutively active S422DSGK1 (sgk-SD) or the inactive K127NSGK1 mutant (sgk-KN). Right: peak Ca2+ concentration after Ca2+ entry in Ca2+-depleted fibroblasts from wild-type mice (sgk1+/+, open bars) or SGK1 knockout mice (sgk1-/-, solid bars) fibroblasts. Ca2+ entry in sgk1-/- fibroblasts is not sensitive to serum deprivation or to dexamethasone plus IGF-I (Shumilina E. et al, (2005) J Cell Physiol). A common SGK1 gene variant is associated with increased blood pressure and body weight. SGK1 may thus contribute to metabolic syndrome. SGK1 may further participate in tumor growth, neurodegeneration, fibrosing disease, and the sequelae of ischemia. SGK3 is required for adequate hair growth and maintenance of intestinal nutrient transport and influences locomotive behavior. In conclusion, SGK1 cover a wide variety of physiological functions and may play an active role in a multitude of pathophysiological conditions. There is little doubt that further targets will be identified that is modulated by the SGK and that further SGK-dependent in vivo physiological functions and pathophysiological conditions will be defined. [...] SGK3 is expressed in all tissues tested thus far and is particularly high in the embryo, adult heart and spleen. Expression of SGK2 is most abundant in epithelial tissues including kidney, liver, pancreas, and presumably choroid plexus of the brain. The subcellular distribution may be nuclear and cytoplasmic, as SGK2 and SGK3 contain a similar nuclear localization signal sequence as SGK1. |
A common (~5% prevalence) SGK1 gene variant is associated with increased blood pressure and body weight. SGK1 may thus contribute to metabolic syndrome. SGK1 may further participate in tumor growth, neurodegeneration, fibrosing disease, and the sequelae of ischemia. SGK3 is required for adequate hair growth and maintenance of intestinal nutrient transport and influences locomotive behavior. In conclusion, the SGKs cover a wide variety of physiological functions and may play an active role in a multitude of pathophysiological conditions. There is little doubt that further targets will be identified that are modulated by the SGK isoforms and that further SGK-dependent in vivo physiological functions and pathophysiological conditions will be defined.
[...] SGK3 is expressed in all tissues tested thus far (172) and is particularly high in the embryo (152, 193) and adult heart and spleen (172). Expression of SGK2 is most abundant in epithelial tissues including kidney, liver, pancreas, and presumably choroid plexus of the brain (172). The subcellular distribution may be nuclear and cytoplasmic, as SGK2 and SGK3 contain a similar nuclear localization signal sequence as SGK1 (112). [page 1160] FIG. 3. Middle: putative role of SGK1 in the regulation of K+ and Ca2+ channels required for stimulation of cell proliferation. Mitogenic factors stimulate the Ca2+ release-activated Ca2+ channel ICRAC. Ca2+ entry through this channel is highly sensitive to cell membrane potential, which is maintained by K+ channels. The stimulation of the voltage-sensitive K+ channel Kv1.3 by SGK1 may serve to maintain the cell membrane polarization and thus sustain oscillating Ca2+ entry through ICRAC. Left: current generated by depolarization of HEK cells overexpressing constitutively active S422DSGK1 (sgk-SD) or the inactive K127NSGK1 mutant (sgk-KN). Right: peak Ca2+ concentration after Ca2+ entry in Ca2+-depleted fibroblasts from wild-type mice (sgk1+/+, open bars) or SGK1 knockout mice (sgk1-/-, solid bars) fibroblasts. Ca2+ entry in sgk1-/- fibroblasts is not sensitive to serum deprivation or to dexamethasone + IGF-I. [Data modified from Shumilina et al. (293).] 112. Firestone GL, Giampaolo JR, and O’Keeffe BA. Stimulus-dependent regulation of the serum and glucocorticoid inducible protein kinase (Sgk) transcription, subcellular localization and enzymatic activity. Cell Physiol Biochem 13: 1–12, 2003. 152. Huber SM, Friedrich B, Klingel K, Lenka N, Hescheler J, and Lang F. Protein and mRNA expression of serum and glucocorticoid-dependent kinase 1 in metanephrogenesis. Dev Dyn 221: 464–469, 2001. 172. Kobayashi T, Deak M, Morrice N, and Cohen P. Characterization of the structure and regulation of two novel isoforms of serumand glucocorticoid-induced protein kinase. Biochem J 344: 189–197, 1999. 193. Lee E, Lein ES, and Firestone GL. Tissue-specific expression of the transcriptionally regulated serum and glucocorticoid-inducible protein kinase (Sgk) during mouse embryogenesis. Mech Dev 103: 177–181, 2001. 293. Shumilina E, Lampert A, Lupescu A, Myssina S, Strutz-Seebohm N, Henke G, Grahammer F, Wulff P, Kuhl D, and Lang F. Deranged Kv channel regulation in fibroblasts from mice lacking the serum and glucocorticoid inducible kinase SGK1. J Cell Physiol 204: 87–98, 2005. |
The source is not given. Note that the paper Shumilina E. et al, (2005) does not contain the figure and its caption. |
|
| [18.] Dsa/Fragment 025 01 - Diskussion Bearbeitet: 8. August 2016, 11:19 WiseWoman Erstellt: 17. May 2015, 22:21 (Hindemith) | Dsa, Fragment, Gesichtet, KomplettPlagiat, Lang et al 2006, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 25, Zeilen: 1ff (entire page) |
Quelle: Lang et al 2006 Seite(n): 1151, 1152, 1163, Zeilen: 1151: last lines, 1152: 1ff; 1163: figure |
|---|---|
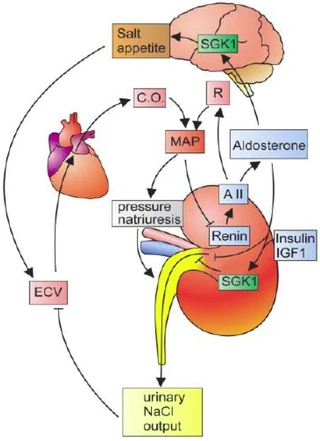
Figure nr. 3 - Dual role of SGK1 in the maintenance of salt homeostasis and blood pressure. SGK1 plays a dual role in the regulation of salt balance, i.e., in the stimulation of both renal Na+ reabsorption and salt appetite. SGK1 contributes to aldosterone- and insulin-induced stimulation of renal Na+ reabsorption. The increased extracellular fluid volume (ECV) enhances the cardiac output (C.O.), thus increasing mean arterial blood pressure (MAP). The enhanced blood pressure leads to pressure natriuresis and thus secondarily increases renal salt excretion, eventually counteracting renal salt retention. A II, angiotensin II; R, total peripheral vascular resistance. SGK1 participate in the regulation of transport, hormone release, neuroexcitability, cell proliferation, and apoptosis. SGK1 contributes to Na+ retention and K+ elimination of the kidney, mineralocorticoid stimulation of salt appetite, glucocorticoid stimulation of intestinal Na+/H+ exchanger and nutrient transport, insulin-dependent salt sensitivity of blood pressure and salt sensitivity of peripheral glucose uptake, memory consolidation, and cardiac repolarization. |
SGKs participate in the regulation of transport, hormone release, neuroexcitability, cell proliferation, and apoptosis. SGK1 contributes to Na+ retention and K+ elimination of the kidney,
[page 1152] mineralocorticoid stimulation of salt appetite, glucocorticoid stimulation of intestinal Na+/H+ exchanger and nutrient transport, insulin-dependent salt sensitivity of blood pressure and salt sensitivity of peripheral glucose uptake, memory consolidation, and cardiac repolarization. [page 1163] FIG. 5. Dual role of SGK1 in the maintenance of salt homeostasis and blood pressure. SGK1 plays a dual role in the regulation of salt balance, i.e., in the stimulation of both renal Na+ reabsorption and salt appetite. SGK1 contributes to aldosterone- and insulin-induced stimulation of renal Na+ reabsorption. The increased extracellular fluid volume (ECV) enhances the cardiac output (C.O.), thus increasing mean arterial blood pressure (MAP). The enhanced blood pressure leads to pressure natriuresis and thus secondarily increases renal salt excretion, eventually counteracting renal salt retention. A II, angiotensin II; R, total peripheral vascular resistance. |
The source is not mentioned. |
|
| [19.] Dsa/Fragment 024 01 - Diskussion Bearbeitet: 8. August 2016, 11:14 WiseWoman Erstellt: 18. May 2015, 10:11 (Hindemith) | Dsa, Fragment, Gesichtet, KomplettPlagiat, Lang et al 2006, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 24, Zeilen: 1ff (entire page) |
Quelle: Lang et al 2006 Seite(n): 1151, 1153, Zeilen: 1151: abstract; 1153: l.col: 28ff |
|---|---|
| Moreover, SGK1 transcription is stimulated by an increased cytosolic Ca2+ concentration (Klingel K. et al., (2000) Am J Physiol Gastrointest Liver Physiol) and by nitric oxide (Turpaev K. et al., (2005) Free Radic Biol Med). SGK1 transcript levels are increased by ischemia of brain (Nishida Y. et al., (2004) Brain Res) and kidney (Feng Y. et al., (2006) Kidney Blood Pressure Res). SGK1 expression is decreased during rejection of transplanted kidneys (Velic A. et al., (2005) Am J Transplant).
Similar to its isoforms SGK2 and SGK3, SGK1 is activated by insulin and growth factors via phosphatidylinositol 3-kinase and the 3-phosphoinositide-dependent kinase PDK1. SGKs activate ion channels (e.g.: ENaC, TRPV5, ROMK, Kv1.3, KCNE1/KCNQ1, GluR1, GluR6), carriers (e.g., NHE3, GLUT1, SGLT1, EAAT1–5), and the Na+-K+-ATPase. They regulate the activity of enzymes (e.g., glycogen synthase kinase-3, ubiquitin ligase Nedd4–2, phosphomannose mutase-2) and transcription factors (e.g., forkhead transcription factor FKHRL1, α-catenin, nuclear factor kB). Leukocyte SGK1 transcript levels are enhanced by treatment with dialysis (Friedrich B. et al., (2005) Nephrol Dial Transplant). A striking increase of SGK1 expression is observed during wound healing (Iyer V. et al., (1999) Science) and in fibrosing tissue, such as diabetic nephropathy (Kumar J. M et al., (1999) J Am Soc Nephrol), glomerulonephritis (Friedrich B. et al., (2002) Kidney Blood Press Res), liver cirrhosis (Fillon S. et al., (2002) Cell Physiol Biochem), fibrosing pancreatitis (Klingel K. et al., (2000) Am J Physiol Gastrointest Liver Physiol), Crohn’s disease (Waldegger S. et al., (1999) Gastroenterology), lung fibrosis and cardiac fibrosis (Vallon V. et al., (2006) J Mol Med). SGK1 gene transcription is stimulated by DNA damage through p53 and activation of extracellular signalregulated kinase (ERK1/2) (Mizuno H. et al., (2001) Genes Cells; You H. et al., (2004) Proc Natl Acad Sci USA), and is also upregulated after neuronal injury (Imaizumi K. et al., (1994) Brain Res), neuronal excitotoxicity (Hollister R. et al., (1997) Neuroscience), and neuronal challenge by exposure to microgravity (David S. et al., (2005) J Neurosci). The promoter of the rat SGK1 gene carries several putative and confirmed transcription factor binding sites including those for the glucocorticoid receptor (GR), the mineralocorticoid receptor (MR), the progesterone receptor (PR), the vitamin D receptor (VDR), the retinoid X receptor (RXR), the farnesoid X receptor (FXR), the sterol regulatory element binding protein (SREBP), PPARγ, the cAMP response element binding protein (CREB), the p53 tumor suppressor protein, the Sp1 transcription factor, the activating protein 1 (AP1), the activating transcription factor 6 (ATF6), the heat shock factor (HSF), reticuloendotheliosis viral oncogene homolog (c-Rel) and nuclear factor κB (NFκB), signal transducers and activators of transcription (STAT), the TGF-α-dependent transcription factors SMAD3 and SMAD4, and forkhead activin signal transducer (FAST) (Firestone G. et al., (2003) Cell Physiol Biochem). The regulation of SGK1 transcript levels is fast; appearance and disappearance of SGK1 mRNA require circa 20 min (Waldegger S. et al., (1997) Proc Natl Acad Sci USA). |
[page 1151]
Similar to its isoforms SGK2 and SGK3, SGK1 is activated by insulin and growth factors via phosphatidylinositol 3-kinase and the 3-phosphoinositide-dependent kinase PDK1. SGKs activate ion channels (e.g., ENaC, TRPV5, ROMK, Kv1.3, KCNE1/KCNQ1, GluR1, GluR6), carriers (e.g., NHE3, GLUT1, SGLT1, EAAT1–5), and the Na+-K+-ATPase. They regulate the activity of enzymes (e.g., glycogen synthase kinase-3, ubiquitin ligase Nedd4–2, phosphomannose mutase-2) and transcription factors (e.g., forkhead transcription factor FKHRL1, α-catenin, nuclear factor κB). [page 1153] Moreover, SGK1 transcription is stimulated by an increased cytosolic Ca2+ concentration (170) and by nitric oxide (321). SGK1 transcript levels are increased by ischemia of brain (236) and kidney (108). SGK1 expression is decreased during rejection of transplanted kidneys (329). Leukocyte SGK1 transcript levels are enhanced by treatment with dialysis (116). A striking increase of SGK1 expression is observed during wound healing (164) and in fibrosing tissue, such as diabetic nephropathy (178, 184), glomerulonephritis (118), liver cirrhosis (110), fibrosing pancreatitis (170), Crohn’s disease (352), lung fibrosis (360), and cardiac fibrosis (323). SGK1 gene transcription is stimulated by DNA damage through p53 and activation of extracellular signal-regulated kinase (ERK1/2) (222, 375), and is also upregulated after neuronal injury (159), neuronal excitotoxicity (145), and neuronal challenge by exposure to microgravity (84). The promoter of the rat SGK1 gene carries several putative and confirmed transcription factor binding sites including those for the glucocorticoid receptor (GR), the mineralocorticoid receptor (MR), the progesterone receptor (PR), the vitamin D receptor (VDR), the retinoid X receptor (RXR), the farnesoid X receptor (FXR), the sterol regulatory element binding protein (SREBP), PPARγ, the cAMP response element binding protein (CREB), the p53 tumor suppressor protein, the Sp1 transcription factor, the activating protein 1 (AP1), the activating transcription factor 6 (ATF6), the heat shock factor (HSF), reticuloendotheliosis viral oncogene homolog (c-Rel) and nuclear factor κB (NFκB), signal transducers and activators of transcription (STAT), the TGF-β-dependent transcription factors SMAD3 and SMAD4, and forkhead activin signal transducer (FAST) (112). The regulation of SGK1 transcript levels is fast; appearance and disappearance of SGK1 mRNA require <20 min (349). 84. David S, Stegenga SL, Hu P, Xiong G, Kerr E, Becker KB, Venkatapathy S, Warrington JA, and Kalb RG. Expression of serum- and glucocorticoid-inducible kinase is regulated in an experience-dependent manner and can cause dendrite growth. J Neurosci 25: 7048–7053, 2005. 108. Feng Y, Wang Y, Xiong J, and Lang F. Expression and significance of serum and glucocorticoid inducible kinase-1 in kidney damage following L-NAME induced hypertension. Kidney Blood Pressure Res. In press. 110. Fillon S, Klingel K, Warntges S, Sauter M, Gabrysch S, Pestel S, Tanneur V, Waldegger S, Zipfel A, Viebahn R, Haussinger D, Broer S, Kandolf R, and Lang F. Expression of the serine/threonine kinase hSGK1 in chronic viral hepatitis. Cell Physiol Biochem 12: 47–54, 2002. 112. Firestone GL, Giampaolo JR, and O’Keeffe BA. Stimulus-dependent regulation of the serum and glucocorticoid inducible protein kinase (Sgk) transcription, subcellular localization and enzymatic activity. Cell Physiol Biochem 13: 1–12, 2003 116. Friedrich B, Alexander D, Aicher WK, Duszenko M, Schaub TP, Passlick-Deetjen J, Waldegger S, Wolf S, Risler T, and Lang F. Influence of standard haemodialysis treatment on transcription of human serum- and glucocorticoid-inducible kinase SGK1 and taurine transporter TAUT in blood leukocytes. Nephrol Dial Transplant 20: 768–774, 2005. 118. Friedrich B, Warntges S, Klingel K, Sauter M, Kandolf R, Risler T, Muller GA, Witzgall R, Kriz W, Grone HJ, and Lang F. Up-regulation of the human serum and glucocorticoid-dependent kinase 1 in glomerulonephritis. Kidney Blood Press Res 25: 303–307, 2002. 145. Hollister RD, Page KJ, and Hyman BT. Distribution of the messenger RNA for the extracellularly regulated kinases 1, 2 and 3 in rat brain: effects of excitotoxic hippocampal lesions. Neuroscience 79: 1111–1119, 1997. 159. Imaizumi K, Tsuda M, Wanaka A, Tohyama M, and Takagi T. Differential expression of sgk mRNA, a member of the Ser/Thr protein kinase gene family, in rat brain after CNS injury. Brain Res 26: 189–196, 1994. 164. Iyer VR, Eisen MB, Ross DT, Schuler G, Moore T, Lee JC, Trent JM, Staudt LM, Hudson J Jr, Boguski MS, Lashkari D, Shalon D, Botstein D and Brown PO. The transcriptional program in the response of human fibroblasts to serum. Science 283: 83–87, 1999. 170. Klingel K, Warntges S, Bock J, Wagner CA, Sauter M, Waldegger S, Kandolf R, and Lang F. Expression of cell volume-regulated kinase h-sgk in pancreatic tissue. Am J Physiol Gastrointest Liver Physiol 279: G998–G1002, 2000. 178. Kumar JM, Brooks DP, Olson BA, and Laping NJ. Sgk, a putative serine/threonine kinase, is differentially expressed in the kidney of diabetic mice and humans. J Am Soc Nephrol 10: 2488–2494, 1999. 184. Lang F, Klingel K, Wagner CA, Stegen C, Warntges S, Friedrich B, Lanzendorfer M, Melzig J, Moschen I, Steuer S, Waldegger S, Sauter M, Paulmichl M, Gerke V, Risler T, Gamba G, Capasso G, Kandolf R, Hebert SC, Massry SG, and Broer S. Deranged transcriptional regulation of cell-volume-sensitive kinase hSGK in diabetic nephropathy. Proc Natl Acad Sci USA 97: 8157–8162, 2000. 222. Mizuno H and Nishida E. The ERK MAP kinase pathway mediates induction of SGK (serum- and glucocorticoid-inducible kinase) by growth factors. Genes Cells 6: 261–268, 2001 236. Nishida Y, Nagata T, Takahashi Y, Sugahara-Kobayashi M, Murata A, and Asai S. Alteration of serum/glucocorticoid regulated kinase-1 (sgk-1) gene expression in rat hippocampus after transient global ischemia. Brain Res 123: 121–125, 2004 321. Turpaev K, Bouton C, Diet A, Glatigny A, and Drapier JC. Analysis of differentially expressed genes in nitric oxide-exposed human monocytic cells. Free Radic Biol Med 38: 1392–1400, 2005 323. Vallon V, Wyatt A, Klingel K, Huang DY, Hussain A, Berchtold S, Friedrich B, Grahammer F, BelAiba RS, Görlach A, Wulff P, Daut J, Dalton ND, Ross J Jr, Flögel U, Schrader J, Osswald H, Kandolf R, Kuhl D, and Lang F. SGK1-dependent cardiac CTGF formation and fibrosis following DOCA treatment. J Mol Med 84; 396–404, 2006. 329. Velic A, Gabriels G, Hirsch JR, Schroter R, Edemir B, Paasche S, and Schlatter E. Acute rejection after rat renal transplantation leads to downregulation of Na+ and water channels in the collecting duct. Am J Transplant 5: 1276–1285, 2005. 349. Waldegger S, Barth P, Raber G, and Lang F. Cloning and characterization of a putative human serine/threonine protein kinase transcriptionally modified during anisotonic and isotonic alterations of cell volume. Proc Natl Acad Sci USA 94: 4440–4445, 1997. 352. Waldegger S, Klingel K, Barth P, Sauter M, Rfer ML, Kandolf R, and Lang F. h-Sgk serine-threonine protein kinase gene as transcriptional target of transforming growth factor beta in human intestine. Gastroenterology 116: 1081–1088, 1999. 360. Wärntges S, Klingel K, Weigert C, Fillon S, Buck M, Schleicher E, Rodemann HP, Knabbe C, Kandolf R, and Lang F. Excessive transcription of the human serum and glucocorticoid dependent kinase hSGK1 in lung fibrosis. Cell Physiol Biochem 12: 135–142, 2002. 375. You H, Jang Y, You T, Okada H, Liepa J, Wakeham A, Zaugg K, and Mak TW. p53-dependent inhibition of FKHRL1 in response to DNA damage through protein kinase SGK1. Proc Natl Acad Sci USA"" 101: 14057–14062, 2004. |
The source is not mentioned here. |
|
| [20.] Dsa/Fragment 023 02 - Diskussion Bearbeitet: 7. August 2016, 21:47 WiseWoman Erstellt: 18. May 2015, 11:11 (Hindemith) | BauernOpfer, Dsa, Fragment, Gesichtet, Lang et al 2006, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 23, Zeilen: 2-40 |
Quelle: Lang et al 2006 Seite(n): 1152, 1153, Zeilen: 1152: l.col: 2ff, 1153: l.col: 1ff |
|---|---|
| SGK1 was originally cloned as an immediate early gene transcriptionally stimulated by serum and glucocorticoids in rat mammary tumor cells (Firestone G. et al., (2003) Cell Physiol Biochem). The human isoform has been discovered as a cell volume regulated gene upregulated by cell shrinkage.
Transcription of SGK1 is upregulated by both serum and glucocorticoids. Several other hormones and mediators stimulate SGK1 transcription, including mineralocorticoids, gonadotropins (Lang F. et al., (2006) Physiol Rev), 1.25-dihydroxyvitamin D3 [sic] (1.25(OH)2D3), transforming growth factor-α (TGF-α) (Kumar JM. et al., (1999) J Am Soc Nephrol; Lang F. et al., (2000) Proc Natl Acad Sci USA), interleukin-6 (Mc Ewen BS. et al., (1995) Vitam Horm), fibroblast and platelet-derived growth factor (Mizuno H. et al., (2001) Genes Cell), thrombin (Belaiba R. et al., (2006) Circ Res), endothelin (Wolf Sc. et al., (2006) Biochem Pharmacol), as well as other cytokines (Verenivov A. et al., (2001) Physiol Biochem). Moreover, activation of peroxisome proliferator-activated receptor γ (PPAR γ) stimulates SGK1 gene transcription (Hong G. et al., (2003) Faseb J). The human isoform has been identified as a cell volume-regulated gene that is transcriptionally upregulated by cell shrinkage. In renal epithelial (A6) cells, SGK1 expression is stimulated by cell swelling rather than cell shrinkage (Rozansky DJ. et al., (2002) Am J Physiol Renal Physiol). SGK1 transcription is further stimulated by excessive glucose concentrations (Lang F. et al., (2000) Proc Natl Acad Sci USA; Saad S. et al., (2005) Kidney Int), heat shock, ultraviolet (UV) radiation, and oxidative stress. SGK1 transcription is inhibited by heparin (Delmolino LM. et al., (1997) J Cell Physiol) and by mutations in the gene MECP2, which underlies Rett syndrome (RTT), a disorder with severe mental retardation (Nuber UA. et al., (2005) Hum Mol Genet). Intestinal SGK1 transcription is further regulated by dietary iron (Marzullo L. et al., (2004) Gene). Signaling molecules involved in the transcriptional regulation of SGK1 include protein kinase C (Lang F. et al., (2000) Proc Natl Acad Sci USA; Mizuno H and Nishida E., (2001) Genes Cells), the protein kinase Raf (Mizuno H and Nishida E., (2001) Genes Cells), mammalian mitogen-activated protein kinase (BMK1) (Hayashi M. et al., (2001) J Biol Chem), mitogen-activated protein kinase (MKK1) (Davies SP. et al., (2000) Biochem J; Mizuno H and Nishida E., (2001) Genes Cells), stress-activated protein kinase-2 (SAPK2, p38 kinase) (Bell LM. et al., (2000) J Biol Chem; Chen S. et al., (2004) Hypertension; Waldegger S. et al., (2000) Cell Physiol Biochem) and phosphatidylinositol (PI) 3-kinase. Follicle-stimulating hormone (FSH) stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid-induced kinase (SGK): evidence for a kinase-independent signaling by FSH in granulosa cells, cAMP (Gonzalez-Robayna IJ. et al., (2000) Mol Endocrinol; Klingel K. et al., (2000) Am J Physiol Gastrointest Liver Physiol) and p53 (Maiyar AC. et al., (1996) J Biol Chem; Maiyar AC. et al., (1997) Mol Endocrinol). |
[page 1152]
The serum- and glucocorticoid-inducible kinase-1 (SGK1) was originally cloned as an immediate early gene transcriptionally stimulated by serum and glucocorticoids in rat mammary tumor cells (112, 361, 362). The human isoform has been discovered as a cell volume-regulated gene upregulated by cell shrinkage (349). [...] [...] Transcription of SGK1 is upregulated by both serum and glucocorticoids (49, 78, 96, 112, 162, 167, 221, 232, 289, 361, 362, 388). Several other hormones and mediators stimulate SGK1 transcription, including mineralocorticoids (29, 49, 65, 96, 135, 168, 197, 206, 231, 290), gonad- [page 1153] otropins (68, 129, 263, 264, 278), 1,25-dihydroxyvitamin D [1,25(OH)2D3] (4), transforming growth factor-β (TGF-β) (178, 184, 352), interleukin-6 (216), fibroblast and plateletderived growth factor (222), thrombin (23), endothelin (366), as well as other cytokines (80, 182, 330). Moreover, activation of peroxisome proliferator-activated receptor γ (PPARγ) stimulates SGK1 gene transcription (146). The human isoform has been identified as a cell volume-regulated gene that is transcriptionally upregulated by cell shrinkage (349). In renal epithelial (A6) cells, SGK1 expression is stimulated by cell swelling rather than cell shrinkage (272). SGK1 transcription is further stimulated by excessive glucose concentrations (184, 275), heat shock, ultraviolet (UV) radiation, and oxidative stress (198). SGK1 transcription is inhibited by heparin (90) and by mutations in the gene MECP2, which underlies Rett syndrome (RTT), a disorder with severe mental retardation (237). Intestinal SGK1 transcription is further regulated by dietary iron (212). Signaling molecules involved in the transcriptional regulation of SGK1 include protein kinase C (184, 222), the protein kinase Raf (222), mammalian mitogen-activated protein kinase (BMK1) (137), mitogen-activated protein kinase (MKK1) (85, 222), stress-activated protein kinase-2 (SAPK2, p38 kinase) (24, 64, 351), phosphatidylinositol (PI) 3-kinase (129), cAMP (129, 170), and p53 (207, 209). [...] 129. Gonzalez-Robayna IJ, Falender AE, Ochsner S, Firestone GL, and Richards JS. Follicle-stimulating hormone (FSH) stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid-lnduced [sic] kinase (Sgk): evidence for A kinase-independent signaling by FSH in granulosa cells. Mol Endocrinol 14: 1283–1300, 2000. [...] 170. Klingel K, Warntges S, Bock J, Wagner CA, Sauter M, Waldegger S, Kandolf R, and Lang F. Expression of cell volume-regulated kinase h-sgk in pancreatic tissue. Am J Physiol Gastrointest Liver Physiol 279: G998–G1002, 2000. [...] 207. Maiyar AC, Huang AJ, Phu PT, Cha HH, and Firestone GL. p53 stimulates promoter activity of the sgk. Serum/glucocorticoid-inducible serine/threonine protein kinase gene in rodent mammary epithelial cells. J Biol Chem 271: 12414–12422, 1996. 209. Maiyar AC, Phu PT, Huang AJ, and Firestone GL. Repression of glucocorticoid receptor transactivation and DNA binding of a glucocorticoid response element within the serum/glucocorticoid-inducible protein kinase (sgk) gene promoter by the p53 tumor suppressor protein. Mol Endocrinol 11: 312–329, 1997 |
The source is mentioned once in the second reference, but without any indication that the entire page is taken from it. For better readability, not all 53 bibliography entries of the source text have been documented. Note also that the title of the publication Gonzalez-Robayna et al. (2000) has made it into the text of the thesis. There is also no entry Gonzales-Robayana et al. (2000) in the thesis bibliography, that author is only listed as second co-author for a different paper published 2000 in a different journal. |
|
| [21.] Dsa/Fragment 022 15 - Diskussion Bearbeitet: 7. August 2016, 21:24 WiseWoman Erstellt: 18. May 2015, 11:39 (Hindemith) | Dsa, Fragment, Gesichtet, KomplettPlagiat, Lang et al 2006, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 22, Zeilen: 15-21 |
Quelle: Lang et al 2006 Seite(n): 1151, 1152, 1153, Zeilen: 1151: last lines; 1152: l.col: 20ff; 1153: r.col: 10ff |
|---|---|
| SGK kinases are expressed in a wide variety of species including shark and Caenorhabditis elegans. Yeast expresses two orthologs, Ypk1 and Ypk2, which are involved in endocytosis and required for survival. Yeast lacking Ypk1 and Ypk2 can be rescued by mammalian SGK1. SGKs participate in the regulation of transport, hormone release, neuroexcitability, cell proliferation, and apoptosis.
Little is known about genomic regulation of SGK2 and SGK3, which appear to be less sensitive to hormonal regulation than SGK1. |
SGKs participate in the regulation of transport, hormone release, neuroexcitability, cell proliferation, and apoptosis.
[page 1152] SGK kinases are expressed in a wide variety of species including shark (348) and Caenorhabditis elegans (142). Yeast express two orthologs, Ypk1 and Ypk2, which are involved in endocytosis (87) and required for survival (60). Yeast lacking Ypk1 and Ypk2 can be rescued by mammalian SGK1 (60). [page 1153] Little is known about genomic regulation of SGK2 and SGK3, which appear to be less sensitive to hormonal regulation than SGK1 (182). 60. Casamayor A, Torrance PD, Kobayashi T, Thorner J, and Alessi DR. Functional counterparts of mammalian protein kinases PDK1 and SGK in budding yeast. Curr Biol 9: 186–197, 1999. 87. DeHart AK, Schnell JD, Allen DA, and Hicke L. The conserved Pkh-Ypk kinase cascade is required for endocytosis in yeast. J Cell Biol 156: 241–248, 2002. 142. Hertweck M, Gobel C, and Baumeister R. C. elegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Dev Cell 6: 577–588, 2004. 182. Lang F and Cohen P. Regulation and physiological roles of serumand glucocorticoid-induced protein kinase isoforms. Sci STKE 2001: RE17, 2001. 348. Waldegger S, Barth P, Forrest JN Jr, Greger R, and Lang F. Cloning of sgk serine-threonine protein kinase from shark rectal gland: a gene induced by hypertonicity and secretagogues. Pflügers Arch 436: 575–580, 1998 |
The source is not mentioned. |
|
| [22.] Dsa/Fragment 040 01 - Diskussion Bearbeitet: 7. August 2016, 21:06 WiseWoman Erstellt: 17. May 2015, 23:45 (Hindemith) | Bowen 2003, Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 40, Zeilen: 1ff (entire page) |
Quelle: Bowen 2003 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| Three organs participate in supplying calcium to blood and removing it from blood when necessary:
• The small intestine is the site where dietary calcium is absorbed. Importantly, efficient absorption of calcium in the small intestine is dependent on expression of a calcium-binding protein in epithelial cells. • Bone serves as a vast reservoir of calcium. Stimulating net resorption of bone mineral releases calcium and phosphate into blood, and suppressing this effect allows calcium to be deposited in bone. • The kidney is critically important in calcium homeostasis. Under normal blood calcium concentrations, almost all of the calcium that enters glomerular filtrate is reabsorbed from the tubular system back into blood, which preserves blood calcium levels. If tubular reabsorption of calcium decreases, calcium is lost by excretion into urine. The following table summarizes body responses to changes in calcium: |
Three organs participate in supplying calcium to blood and removing it from blood when necessary:
• The small intestine is the site where dietary calcium is absorbed. Importantly, efficient absorption of calcium in the small intestine is dependent on expression of a calcium-binding protein in epithelial cells. • Bone serves as a vast reservoir of calcium. Stimulating net resorption of bone mineral releases calcium and phosphate into blood, and suppressing this effect allows calcium to be deposited in bone. • The kidney is critcally [sic] important in calcium homeostasis. Under normal blood calcium concentrations, almost all of the calcium that enters glomerular filtrate is reabsorbed from the tubular system back into blood, which preserves blood calcium levels. If tubular reabsorption of calcium decreases, calcium is lost by excretion into urine. [...] [...] The following table summarizes body responses to conditions that would otherwise lead to serious imbalances in calcium and phosphate levels in blood. |
The source is not given. |
|
| [23.] Dsa/Fragment 039 09 - Diskussion Bearbeitet: 7. August 2016, 21:02 WiseWoman Erstellt: 17. May 2015, 23:35 (Hindemith) | Bowen 2003, Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 39, Zeilen: 9-25 |
Quelle: Bowen 2003 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| It is critical to maintain blood calcium concentrations within a tight normal range. Deviations above or below the normal range frequently lead to serious diseases. There are three major pools of calcium in the body:
• Intracellular calcium: a large majority of calcium within cells is sequestered in mitochondria and endoplasmic reticulum. Intracellular free calcium concentrations fluctuate greatly, from roughly 100 nM to greater than 1 μM, due to release from cellular stores or influx from extracellular fluid. These fluctuations are integral to calcium's role in intracellular signaling, enzyme activation and muscle contractions. • Calcium in blood and extracellular fluid: roughly half of the calcium in blood is bound to proteins. The concentration of ionized calcium in this compartment is normally almost invariant at approximately 1 mM or 10000 times the basal concentration of free calcium within cells. Also, the concentration of phosphorus in blood is essentially identical to that of calcium. • Bone calcium: a vast majority of body calcium is in bone. Within bone, 99% of the calcium is tied up in the mineral phase, but the remaining 1% is in a pool that can rapidly exchange with extracellular calcium. |
It is critical to maintain blood calcium concentrations within a tight normal range. Deviations above or below the normal range frequently lead to serious disease.
[...] There are three major pools of calcium in the body: • Intracellular calcium: A large majority of calcium within cells is sequestered in mitochondria and endoplasmic reticulum. Intracellular free calcium concentrations fluctuate greatly, from roughly 100 nM to greater than 1 uM, due to release from cellular stores or influx from extracellular fluid. These fluctuations are integral to calcium's role in intracellular signaling, enzyme activation and muscle contractions. • Calcium in blood and extracellular fluid: Roughly half of the calcium in blood is bound to proteins. The concentration of ionized calcium in this compartment is normally almost invariant at approximately 1 mM, or 10,000 times the basal concentration of free calcium within cells. Also, the concentration of phosphorus in blood is essentially identical to that of calcium. • Bone calcium: A vast majority of body calcium is in bone. Within bone, 99% of the calcium is tied up in the mineral phase, but the remaining 1% is in a pool that can rapidly exchange with extracellular calcium. |
The source is not mentioned. |
|
| [24.] Dsa/Fragment 041 04 - Diskussion Bearbeitet: 7. August 2016, 12:09 WiseWoman Erstellt: 18. May 2015, 17:50 (Hindemith) | Calcium 2005, Dsa, Fragment, Gesichtet, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 41, Zeilen: 4-6, 9-27 |
Quelle: Calcium 2005 Seite(n): 19, 21, 22, 23, Zeilen: Slides 19: 2ff; 21: 1ff; 22: 1ff; 23: 1ff |
|---|---|
| PTH has little effect on modulating calcium fluxes in the proximal tubule where 65% of the filtered calcium is reabsorbed, by being coupled to the transport of solutes such as sodium and water. [...] About 20% of filtered calcium is reabsorbed in the cortical thick ascending limb of the loop of Henle (CTAL) and 15% in the distal convoluted tubule (DCT). Here PTH also binds to the PTHR and again by a cyclic AMP-mediated mechanism, enhances calcium reabsorption. In the CTAL, this appears to occur by increasing the activity of the Na+-K+-2Cl- cotransporter that drives NaCl reabsorption and also stimulates paracellular calcium and magnesium reabsorption.
The extracellular calcium sensor is also resident in the CTAL and can respond to increased ECF calcium by activating phospholipase A2, reducing the activity of the Na+-K+-2Cl- cotransporter and of an apical K+ channel, and diminishing paracellular calcium and magnesium reabsorption. Consequently raised ECF calcium antagonizes the effect of PTH in this region of the kidney and ECF calcium can regulate its own homeostasis. In the DCT, PTH can also influence transcellular calcium transport. This is a multistep process involving transfer of luminal Ca2+ into the renal tubule cell via the transient receptor potential channel (TRPV5) or ECaC (epithelial calcium channel), translocation of Ca2+ across the cell from apical to basolateral surface a process involving proteins such as calbindin- D28K and finally active extrusion of Ca2+ from the cell into the blood via a Na+-Ca2+ exchanger, designated NCX1 and the Ca2+-ATPase pump. PTH markedly stimulates Ca2+ reabsorption in the DCT primarily by augmenting NCX1 activity via a cyclic AMP-mediated mechanism. |
[slide 19]
PTH has little effect on modulating calcium fluxes in the proximal tubule where 65% of the filtered calcium is reabsorbed, coupled to the bulk transport of solutes such as sodium and water. [slide 21] About 20% of filtered calcium is reabsorbed in the cortical thick ascending limb of the loop of Henle (CTAL) and 15% in the distal convoluted tubule (DCT) and it is here that PTH also binds to the PTHR and again by a cyclic AMP-mediated mechanism, enhances calcium reabsorption. In the CTAL, at least, this appears to occur by increasing the activity of the Na/K/2Cl cotransporter that drives NaCl reabsorption and also stimulates paracellular calcium and magnesium reabsorption. [slide 22] The CaSR is also resident in the CTAL and can respond to an increased ECF calcium by activating phospholipase A2, reducing the activity of the Na/K/2Cl cotransporter and of an apical K channel, and diminishing paracellular calcium and magnesium reabsorption. Consequently a raised ECF calcium antagonizes the effect of PTH in this nephron segment and ECF calcium can in fact participate in this way in the regulation of its own homeostasis. [slide 23] In the distal convoluted tubule (DCT), PTH can also influence transcellular calcium transport. This is a multistep process involving
PTH markedly stimulates Ca2+ reabsorption in the DCT primarily by augmenting NCX1 activity via a cyclic AMP-mediated mechanism |
No source is given for this passage. The source is probably not the PowerPoint slide set but the online textbook that was first published in 2000. However, the book is constantly updated and not archived on the Internet Archive, so this is the only source. Both PowerPoint and textbook do not use superscripts. |
|
| [25.] Dsa/Fragment 045 01 - Diskussion Bearbeitet: 7. August 2016, 10:40 WiseWoman Erstellt: 17. May 2015, 21:15 (Hindemith) | Dsa, Fragment, Gesichtet, Hoenderop et al 2003, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 45, Zeilen: 1-5 |
Quelle: Hoenderop et al 2003 Seite(n): 1907, Zeilen: l.col: 19ff |
|---|---|
| TRPV5 and TRPV6 are Ca2+- selective channels that belong, together with the temperature-activated vanilloid receptors, to the TRPV subfamily. Genomic cloning has demonstrated that TRPV5 and TRPV6 form a unique pair of novel Ca2+ channels that are transcribed from distinct genes juxtaposed on human chromosome 7q35. | TRPV5 and TRPV6 are Ca2+-selective channels that belong, together with the temperature-activated vanilloid receptors, to the TRPV subfamily (3, 4). Genomic cloning has demonstrated that TRPV5 and TRPV6 form a unique pair of novel Ca2+ channels that are transcribed from distinct genes juxtaposed on human chromosome 7q35 (4).
3. Montell, C., Birnbaumer, L., and Flockerzi, V. 2002. The TRP channels, a remarkably functional family. Cell. 108:595–598. 4. Hoenderop, J.G., Nilius, B., and Bindels, R.J. 2002. Molecular mechanisms of active Ca2+ reabsorption in the distal nephron. Ann. Rev. Physiol. 64:529–549. |
The source is not mentioned. The passage starts on the previous page Dsa/044. |
|
| [26.] Dsa/Fragment 044 15 - Diskussion Bearbeitet: 7. August 2016, 10:36 WiseWoman Erstellt: 17. May 2015, 21:12 (Hindemith) | Dsa, Fragment, Gesichtet, Hoenderop et al 2003, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 44, Zeilen: 15-47 |
Quelle: Hoenderop et al 2003 Seite(n): 1906, 1907, Zeilen: 1906: abstract, r.col: 10ff; 1907: l.col: 1ff |
|---|---|
| Transient receptor potential (TRP) cation channel subfamily V, members 5 and 6 (TRPV5 and TRPV6) have recently been postulated to be the molecular gatekeepers facilitating Ca2+ influx in these tissues and are members of the TRP family, which mediates diverse biological effects ranging from pain perception to male aggression. Genetic ablation of TRPV5 in the mouse allowed us to investigate the function of this novel Ca2+ channel in maintaining the Ca2+ balance. Here, we demonstrate that mice lacking TRPV5 display diminished active Ca2+ reabsorption despite enhanced vitamin D levels, causing severe hypercalciuria. In vivo micropuncture experiments demonstrated that Ca2+ reabsorption was malfunctioning within the early part of the distal convolution, exactly where TRPV5 is localized. In addition, compensatory hyperabsorption of dietary Ca2+was measured in TRPV5 knockout mice. Furthermore, the knockout mice exhibited significant disturbances in bone structure, including reduced trabecular and cortical bone thickness. These data demonstrate the key function of TRPV5 in active Ca2+ reabsorption and its essential role in the Ca2+ homeostasis.
In humans, the daily dietary Ca2+ intake is less than 1000 mg, of which only 30% is absorbed in the intestinal tract. This percentage is significantly enhanced during growth, pregnancy, and lactation by increased levels of circulating 1.25-(OH)2D3. Although there is continuous turnover of bone mass, there is no net gain or loss of Ca2+ from bone in a young and healthy individual. This indicates that healthy adults excrete a maximum of 300 mg Ca2+ in the urine to balance the intestinal Ca2+ uptake and that the remaining 98% of the Ca2+ filtered in the glomeruli is reabsorbed along the nephron. The molecular mechanism responsible for Ca2+ absorption in the small intestine and the kidney was elusive for a long time. The cloning of transient receptor potential (TRP) cation channel subfamily V, member 5 (TRPV5; originally called ECaC1) from vitamin D–responsive rabbit renal epithelial cells and TRP cation channel subfamily V, member 6 (TRPV6; originally called CaT1) from rat duodenum has ignited research into transcellular Ca2+ (re)absorption at the molecular level. Mammals harbor at least 21 genes of the so-called TRP channels, whose functions remain mostly unknown. TRPV5 has been implicated as the Ca2+ influx channel in the process of vitamin D–responsive active Ca2+ reabsorption in the kidney. In comparison, the TRPV5 homolog TRPV6, which displays an amino acid sequence identity of about 75% to TRPV5, has been postulated to be the Ca2+ influx channel facilitating Ca2+ absorption in enterocytes. TRPV6 is ubiquitously expressed and has been implicated as part of the capacitative Ca2+ [entry mechanism and, therefore, intracellular Ca2+ signaling.] |
Transient receptor potential (TRP) cation channel subfamily V, members 5 and 6 (TRPV5 and TRPV6) have recently been postulated to be the molecular gatekeepers facilitating Ca2+ influx in these tissues and are members of the TRP family, which mediates diverse biological effects ranging from pain perception to male aggression. Genetic ablation of TRPV5 in the mouse allowed us to investigate the function of this novel Ca2+ channel in maintaining the Ca2+ balance. Here, we demonstrate that mice lacking TRPV5 display diminished active Ca2+ reabsorption despite enhanced vitamin D levels, causing severe hypercalciuria. In vivo micropuncture experiments demonstrated that Ca2+ reabsorption was malfunctioning within the early part of the distal convolution, exactly where TRPV5 is localized. In addition, compensatory hyperabsorption of dietary Ca2+ was measured in TRPV5 knockout mice. Furthermore, the knockout mice exhibited significant disturbances in bone structure, including reduced trabecular and cortical bone thickness. These data demonstrate the key function of TRPV5 in active Ca2+ reabsorption and its essential role in the Ca2+ homeostasis.
[...] [...] In humans, the daily dietary Ca2+ intake is less than 1,000 mg, of which only 30% is absorbed in the intestinal tract. This percentage is significantly enhanced during growth, pregnancy, and lactation by increased levels of circulating 1,25-(OH)2D3. Although there is continuous turnover of bone mass, there is no net gain or loss of Ca2+ from bone in a young and healthy individual. This indicates that healthy adults excrete a maximum of 300 mg Ca2+ in the urine to balance the intestinal Ca2+ uptake and that the remaining 98% of the Ca2+ filtered in the glomeruli is reabsorbed along the nephron. The molecular mechanism responsible for Ca2+ absorption in the small intestine and the kidney was elusive for a long time. [page 1907] The cloning of transient receptor potential (TRP) cation channel subfamily V, member 5 (TRPV5; originally called ECaC1) from vitamin D–responsive rabbit renal epithelial cells (1) and TRP cation channel subfamily V, member 6 (TRPV6; originally called CaT1) from rat duodenum (2) has ignited research into transcellular Ca2+ (re)absorption at the molecular level (1). Mammals harbor at least 21 genes of the so-called TRP channels, whose functions remain mostly unknown (3). TRPV5 has been implicated as the Ca2+ influx channel in the process of vitamin D–responsive active Ca2+ reabsorption in the kidney (1, 4). In comparison, the TRPV5 homolog TRPV6, which displays an amino acid sequence identity of about 75% to TRPV5, has been postulated to be the Ca2+ influx channel facilitating Ca2+ absorption in enterocytes (2). TRPV6 is ubiquitously expressed and has been implicated as part of the capacitative Ca2+ entry mechanism and, therefore, intracellular Ca2+ signaling (5). 1. Hoenderop, J.G., et al. 1999. Molecular identification of the apical Ca2+ channel in 1, 25-dihydroxyvitamin D3-responsive epithelia. J. Biol. Chem. 274:8375–8378. 2. Peng, J.B., et al. 1999. Molecular cloning and characterization of a channel- like transporter mediating intestinal calcium absorption. J. Biol. Chem. 274:22739–22746. 3. Montell, C., Birnbaumer, L., and Flockerzi, V. 2002. The TRP channels, a remarkably functional family. Cell. 108:595–598. 4. Hoenderop, J.G., Nilius, B., and Bindels, R.J. 2002. Molecular mechanisms of active Ca2+ reabsorption in the distal nephron. Ann. Rev. Physiol. 64:529–549. 5. Yue, L., Peng, J.B., Hediger, M.A., and Clapham, D.E. 2001. CaT1 manifests the pore properties of the calcium-release-activated calcium channel. Nature. 410:705–709. |
The source is not mentioned. |
|
| [27.] Dsa/Fragment 018 20 - Diskussion Bearbeitet: 7. August 2016, 10:17 WiseWoman Erstellt: 17. May 2015, 18:04 (Hindemith) | Dsa, Fragment, Gesichtet, SMWFragment, Schutzlevel sysop, Verschleierung, Wikipedia Serine-threonine-specific protein kinase 2006 |
|
|
| Untersuchte Arbeit: Seite: 18, Zeilen: 20-39 |
Quelle: Wikipedia Serine-threonine-specific protein kinase 2006 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| 1) Serine/threonine-specific protein kinases
Serine/threonine protein kinases phosphorylate the -OH group of serine or threonine (which has similar sidechains). Activity of these protein kinases can be regulated by specific events (e.g. DNA damage), as well as numerous chemical signals, including cAMP/cGMP, diacylglycerol, and Ca2+/calmodulin. These kinases are not specific to a similar consensus sequence - that is there is no common "target sequence" to be phosphorylated. Since the substrate to be phosphorylated aligns with the kinase by several key amino acids (usually through hydrophopic forces and ionic bonds), a kinase is usually specific, not to a single substrate, but to a whole "substrate family" having common properties. The kinases are usually inactivated by a pseudosubstrate that binds to the kinase like a real substrate but lacks the amino acid to be phosphorylated. Its removal activates the kinase. The catalytic domain of these kinases is highly conserved. - Phosphorylase kinase: phosphorylase kinase was the first Ser/Thr protein kinase to be discovered (Krebs EG et al., (1959) J Biol Chem). - Protein kinase A: protein kinase A consists of two domains, a small domain with several β sheet structures and a larger domain containing several α helices. The binding sites for substrate and ATP are located in the catalytic cleft between the domains (or lobes). When ATP and substrate bind, the two lobes rotate so that the terminal phosphate group of the ATP and the target amino acid of the substrate move into the correct positions for the catalytic reaction to take place. |
Serine/threonine-specific protein kinase
[...] Serine/threonine protein kinases (EC 2.7.11.1) phosphorylate the OH group of serine or threonine (which have similar sidechains). Activity of these protein kinases can be regulated by specific events (e.g. DNA damage), as well as numerous chemical signals, including:
While serine/threonine kinases all phosphorylate serine or threonine residues in their substrates, they select specific residues to phosphorylate on the basis of residues that flank the phosphoacceptor site, which together comprise the consensus sequence. Since the consensus sequence residues of the substrate to be phosphorylated make contact with the catalytic cleft of the kinase at several key amino acids (usually through hydrophobic forces and ionic bonds), a kinase is usually not specific to a single substrate, but instead can phosphorylate a whole "substrate family" having common recognition sequences. While the catalytic domain of these kinases is highly conserved, the sequence variation that is observed in the kinome (the subset of genes in the genome that encode kinases) provides for recognition of distinct substrates. Most kinases are inhibited by a pseudosubstrate that binds to the kinase like a real substrate but lacks the amino acid to be phosphorylated. When the pseudosubstrate is removed, the kinase can perform its normal function. [...] Phosphorylase kinase Phosphorylase kinase (EC 2.7.11.19) was in fact, the first Ser/Thr protein kinase to be discovered (in 1959 by Krebs et al.). Protein kinase A [...] Protein kinase A (EC 2.7.11.1) consists of two domains, a small domain with several β sheet structures and a larger domain containing several α helices. The binding sites for substrate and ATP are located in the catalytic cleft between the domains (or lobes). When ATP and substrate bind, the two lobes rotate so that the terminal phosphate group of the ATP and the target amino acid of the substrate move into the correct positions for the catalytic reaction to take place. |
No source is given. |
|
| [28.] Dsa/Fragment 088 21 - Diskussion Bearbeitet: 6. August 2016, 20:50 WiseWoman Erstellt: 17. May 2015, 20:03 (Hindemith) | Boini 2006, Dsa, Fragment, Gesichtet, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 88, Zeilen: 21-35 |
Quelle: Boini 2006 Seite(n): 85, 87, Zeilen: 85: 9-19; 87: 15ff |
|---|---|
| Enhanced SGK1 expression has been observed in the salt-sensitive Dahl rat (Farjah M. et al., (2003) Hypertension), and moderately enhanced blood pressure is observed in individuals carrying a variant of the SGK1 gene, affecting as many as 5% of unselected Caucasians (Busjahn A. et al., (2002) Hypertension). In the same individuals, increased body mass index (Dieter M. et al., (2004) Obes Res) and a shortening of the Q-T interval (Busjahn A. et al., (2002) Hypertension; Busjahn A and Luft FC., (2003) Cell Physiol Biochem) have been observed. The increased body mass index may be partially due to enhanced stimulation of the intestinal glucose transporter SGLT1 (Dieter M. et al., (2004) Obes Res), the accelerated cardiac repolarization due to enhanced activation of the cardiac K+ channel KCNE1 (Busjahn A. et al., (2004) Cell Physiol Biochem; Embark HM. et al., (2004) Cell Physiol Biochem). Thus excessive stimulation of carriers and channels by SGK1 could account for obesity, hypertension, and shortened cardiac action potential. SGK1 has previously been shown to regulate the ROMK1 channel (Yun CC et al., (2002) J Biol Chem). Moreover, SGK1-dependent ENaC activity is expected to depolarize the apical cell membrane of principal cells, thus favoring K+ secretion. | Along those lines enhanced SGK1 expression has been observed in the salt sensitive Dahl rat (Farjah., 2003). In addition, moderately enhanced blood pressure is observed in individuals carrying a variant of the SGK1 gene, affecting as many as 5% of unselected Caucasians (Busjahn et al., 2002). In the same individuals increased body mass index and a shortening of the QT interval (Busjahn et al., 2002; Busjahn and Luft 2003) have been observed. The increased body mass index may be partially due to enhanced stimulation of the intestinal glucose transporter SGLT1 (Dieter et al., 2004), the accelerated cardiac repolarization due to enhanced activation of the cardiac K+ channel KCNE1 (Busjahn et al., 2004; Embark et al., 2003). Thus, altered regulation of carriers and channels by SGK1 could account for the coincidence of obesity, hypertension and altered cardiac action potential (Lang et al., 2003). [...]
[page 87] [...] SGK1 has previously been demonstrated to regulate the renal outer medullary K+ channel ROMK1 (Yun et al., 2002), and possibly further K+ channels important for renal K+ elimination. Beyond that, SGK1 dependent activity of ENaC is expected to depolarize the apical cell membrane of principal cells thus favoring K+ secretion. |
The source is not mentioned. |
|
| [29.] Dsa/Fragment 060 29 - Diskussion Bearbeitet: 6. August 2016, 20:45 WiseWoman Erstellt: 17. May 2015, 19:55 (Hindemith) | Boini 2006, Dsa, Fragment, Gesichtet, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 60, Zeilen: 29-37 |
Quelle: Boini 2006 Seite(n): 35, Zeilen: 20ff |
|---|---|
| The tail cuff method has the advantage to be noninvasive and can provide reproducible results of systolic blood pressure if those precautions are taken into account (Kurtz et al., (2005) Arterioscler Tromb Vasc Biol). Systolic arterial blood pressure was determined by the tail-cuff method (IITC, model 179, Woodland Hills, California, USA) before, 7 weeks and 12 weeks following the initiation of DOCA/high-salt treatment. As reviewed recently, (Meneton P et al., (2000) J Am Soc Neprhol), the tail cuff approach to determine arterial blood pressure requires certain precautions to reduce the stress of the animals, including appropriate training of the mice over multiple days and adequate prewarming to dilate the tail artery. | III. 3. Blood pressure: Systolic arterial blood pressure was determined by application of the tail-cuff method. As reviewed recently (Meneton et al., 2000), the tail cuff approach to determine arterial blood pressure requires certain precautions to reduce the stress of the animals, including appropriate training of the mice over multiple days, prewarming to an ambient temperature of 29°C, measurement in a quiet, semidarkend and clean environment, and performance of the measurements by one person and during a defined day time, when blood pressure is stable (between 1-3 PM). All these precautions were taken in the present study. The tail cuff method has the advantage to be noninvasive and can provide reproducible results of systolic blood pressure if those precautions are taken into account (Kurtz et al., 2005). |
The source is not given. |
|
| [30.] Dsa/Fragment 032 00 - Diskussion Bearbeitet: 6. August 2016, 20:40 WiseWoman Erstellt: 17. May 2015, 20:58 (Hindemith) | Dsa, Fragment, Gesichtet, SMWFragment, Schutzlevel sysop, Verschleierung, Wikipedia Epithelial sodium channel 2007 |
|
|
| Untersuchte Arbeit: Seite: 32, Zeilen: figure |
Quelle: Wikipedia Epithelial sodium channel 2007 Seite(n): 1 (online source), Zeilen: - |
|---|---|
Figure nr. 5 - Schematic picture of an ENaC (the second α-subunit is omitted for clarity). |
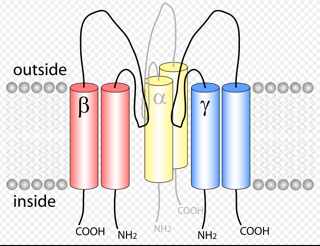
schematic picture of an ENaC (the second α-subunit is omitted for clarity). |
The source is not mentioned, although it clearly has been the basis for the figure in the dissertation. Acording to [1], the figure is available under the CC BY-SA 3.0 licence, which demands that credit be given to the original creator. |
|
| [31.] Dsa/Fragment 055 03 - Diskussion Bearbeitet: 6. August 2016, 20:35 WiseWoman Erstellt: 17. May 2015, 19:51 (Hindemith) | Boini 2006, Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 55, Zeilen: 3-13 |
Quelle: Boini 2006 Seite(n): 34, Zeilen: 4ff |
|---|---|
| Mice deficient in SGK1 (sgk1–/–) were generated and bred as previously described (Huang et al., (2004) J Am Soc Nephol; Wulff et al., (2002) J Clin Invest; Huang et al., (2004) J Am Soc Nephol). In brief, a conditional targeting vector was generated from a 7-kb fragment encompassing the entire transcribed region on 12 exons. The neomycin resistance cassette was flanked by two loxP sites and inserted into intron 11. Exons 4–11, which code for the sgk1 kinase domain, were “floxed” by inserting a third loxP site into intron 3. A clone with a recombination between the first and third loxP site (type I recombination) was injected into C57BL/6 blastocytes. Male chimeras were bred to C57BL/6 and 129/SvJ females. Heterozygous SGK1-deficient mice were backcrossed to 129/SvJ wild-type mice (Charles River, Sulzfeld, Germany) for ten generations and then intercrossed to generate homozygous SGK1 knockout mice (sgk1−/−) and their wild type littermates (sgk1+/+). | Mice deficient in SGK1 (sgk1-/-) were generated and bred as previously described (Wulff et al., 2002; Huang et al., 2004). In brief, a conditional targeting vector was generated from a 7-kb fragment encompassing the entire transcribed region on 12 exons. The neomycin resistance cassette was flanked by two loxP sites and inserted into intron 11. Exons 4–11, which code for the sgk1 kinase domain, were “floxed” by inserting a third loxP site into intron 3. A clone with a recombination between the first and third loxP site (type I recombination) was injected into C57BL/6 blastocytes. Male chimeras were bred to C57BL/6 and 129/SvJ females. Heterozygous SGK1-deficient mice were backcrossed to 129/SvJ wild-type mice (Charles River, Sulzfeld, Germany) for ten generations and then intercrossed to generate homozygous SGK1 knockout mice (sgk1-/-) and their wild type littermates (sgk1+/+). |
The source is not mentioned. It is not surprising that the two dissertations have used mice that have been treated identically, and it is efficient to describe this with the same words, but this should have been made transparent. |
|
| [32.] Dsa/Fragment 039 01 - Diskussion Bearbeitet: 6. August 2016, 20:29 WiseWoman Erstellt: 17. May 2015, 19:47 (Hindemith) | Boini 2006, Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 39, Zeilen: 1-6 |
Quelle: Boini 2006 Seite(n): 21, Zeilen: 2ff |
|---|---|
| The in vivo significance of SGK1 in regulation of renal K+ transport was recently analyzed in SGK1 KO mice. These mice indeed show a disturbed adaptation to an acute and chronic K+ load, but, as indicated by electrophysiological and immunohistochemical data obtained from these mice after a chronic potassium load, this maladaptation likely is related to altered ENaC (or Na+, K+-ATPase) activity in the ASDN cells rather than to inhibition of ROMK cell surface targeting or activity (Huang DY. et al., (2004) J Am Soc Neprhol). | The in vivo significance of SGK1 in regulation of renal K+ transport was recently analyzed in SGK1 KO mice. These mice indeed show a disturbed adaptation to an acute and chronic K+ load, but, as indicated by electrophysiological and immunohistochemical data obtained from these mice after a chronic potassium load, this maladaptation likely is related to altered ENaC (or Na+,K+-ATPase) activity in the ASDN cells rather than to inhibition of ROMK cell surface targeting or activity (Huang et al., 2004). |
The source is not given. |
|
| [33.] Dsa/Fragment 038 01 - Diskussion Bearbeitet: 6. August 2016, 20:25 WiseWoman Erstellt: 17. May 2015, 19:45 (Hindemith) | Boini 2006, Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 38, Zeilen: 1-16 |
Quelle: Boini 2006 Seite(n): 20, 21, Zeilen: 20: 18ff; 21: 1ff |
|---|---|
| Patch-clamp studies on rat CCDs found no measurable effect of acute aldosterone administration on K+ channel number, open probability, or conductance (Palmer LG. et al., (1994) Am J Physiol). However, some data suggested that aldosterone induces renal K+ secretion already at aldosterone concentrations that do not exhibit any measurable effect on urinary Na+ excretion (Bhargava et al., (2001) Endocrinology). Moreover, high K+ intake increases ROMK activity more efficiently in intact rats than in adrenalectomized animals, suggesting that aldosterone may have at least a permissive effect on ROMK activation (Palmer LG. et al., (1994) Am J Physiol).
Consistent with a possible role of aldosterone in ROMK regulation, recent studies in heterologous expression systems advocated a regulatory action of aldosterone-induced SGK1 on ROMK cell surface activity and abundance (Palmada M. et al., (2003) Biochem Biophys Res Commun). The regulatory role of SGK1 with regard to ROMK may be indirect via increased interaction with the Na+, H+ exchanger–regulating factor 2 (NHERF2) (Palmada M. et al., (2003) Biochem Biophys Res Commun) or direct via increased phosphorylation of ROMK at a serine residue within the canonical SGK1 consensus phosphorylation motif (Yoo D. et al., (2003) J Biol Chem). |
Patch-clamp studies on rat CCDs found no measurable effect of acute aldosterone administration on K+ channel number, open probability, or conductance (Palmer et al., 1994). However, some data suggested that aldosterone induces renal K+ secretion already at aldosterone concentrations that do not exhibit any measurable effect on urinary Na+ excretion (Bhargava et al., 2001). Moreover, high K+ intake increases ROMK activity more efficiently in intact rats than in adrenalectomized animals, suggesting that aldosterone may have at least a permissive effect on ROMK activation (Palmer et al., 1994). Consistent with a possible role of aldosterone in ROMK regulation, recent studies in heterologous expression systems advocated a regulatory action of aldosterone-induced SGK1 on ROMK cell surface activity and abundance (Palmada et al., 2003, Palmada et al., 2003a). The regulatory role of SGK1 with regard to ROMK may be indirect via increased interaction with the Na+, H+ exchanger–regulating factor 2 (NHERF2) (Palmada et al., 2003, Palmada et al., 2003a) or direct via
[page 21] increased phosphorylation of ROMK at a serine residue within the canonical SGK1 consensus phosphorylation motif (Yoo et al., 2003). |
The source is not given. |
|
| [34.] Dsa/Fragment 037 01 - Diskussion Bearbeitet: 6. August 2016, 20:21 WiseWoman Erstellt: 17. May 2015, 19:42 (Hindemith) | Boini 2006, Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 37, Zeilen: 1ff (entire page) |
Quelle: Boini 2006 Seite(n): 19, 20, Zeilen: 19: 17ff; 20: 1ff |
|---|---|
| [Under a standard diet, the KO mice have] unaltered Na+ excretion as compared to their wildtype littermates. However, plasma aldosterone levels are significantly increased in sgk1−/− mice, suggesting extracellular volume contraction.
Under dietary Na+ restriction, activated compensatory mechanisms are no longer sufficient to keep the mice in Na+ balance, and mice disclosed significant loss in renal NaCl and in body weight. Experiments on collecting ducts perfused ex vivo revealed significantly lower transepithelial amiloride-sensitive potential differences, consistent with a reduced Na transport activity in the CCD. Although apical localization of ENaC was seen in both Na+- restricted sgk1+/+ and sgk1−/− mice, the apical localization of ENaC is inappropriately low in the sgk1−/− mice given the several fold higher plasma aldosterone levels in the KO mice. Nevertheless, these data, together with the rather mild phenotype of sgk1−/− mice, as compared to the much more severe and life-threatening phenotypes of MR or ENaC ko mice, suggest that (a) aldosterone-dependent control of ENaC function does not solely rely on the induction and activation of SGK1 and (b) some redundancy exists in the signal transduction pathway that controls ENaC activity. Consistent with these ideas, Loffing and his coworkers found significant phosphorylation of the SGK1 target Nedd4-2 in mouse mpkCCDcl4 cells in vitro and in rat collecting ducts in vivo in the absence of any aldosterone and detectable SGK1 protein expression (Flores SY. et al., (2005) J Am Soc Nephrol). In addition to aldosterone-dependent regulation of renal Nareabsorption [sic] , SGK1 appears to be involved also in the regulation of aldosterone-induced salt appetite. Sgk1+/+ and sgk1−/− mice show a similar salt intake under standard conditions. Treatment with the synthetic aldosterone analogue deoxycorticosterone-acetate (DOCA) increases Na+ intake much more in sgk1+/+ mice than in sgk1−/− mice. The underlying mechanism for the reduced mineralocorticoid-induced salt intake is unclear (Vallon V. et al., (2005) Am J Physiol Regul Integr Comp Physiol). b) Role of SGK1 in renal K+ secretion Aside from its stimulatory effect on renal Na+ reabsorption, aldosterone has strong kaliuretic action. Renal K+ secretion also takes place in the ASDN and is likely mediated by the renal outer medullary K+ channel ROMK. ROMK is coexpressed with ENaC in the ASDN cells, and Na+ reabsorption via ENaC provides the necessary driving force for K+ secretion. Consistently, pharmacological inhibition (i.e., by amiloride) or genetic loss of function (i.e., pseudohypoaldosteronism (PHA) type 1) of ENaC lower renal K+ secretion and predispose one to hyperkalemia. It remains unresolved whether the kaliuretic effect of aldosterone is entirely secondary to the activation of ENaC-mediated Na+ reabsorption or whether aldosterone directly regulates ROMK function. |
Under a standard diet, the KO mice have unaltered Na+ excretion as compared to their wildtype littermates. However, plasma aldosterone levels are significantly increased in sgk1-/- mice, suggesting extracellular volume contraction. Under dietary Na+ restriction, activated compensatory mechanisms are no longer sufficient to keep the mice in Na+ balance, and mice disclosed significant loss in renal NaCl and in body weight. Experiments on collecting ducts perfused ex vivo revealed significantly lower transepithelial amiloride-sensitive potential differences, consistent with a reduced Na+ transport activity in the CCD. Although apical localization of ENaC was seen in both Na+-restricted sgk+/+ [sic] and sgk1-/- mice, the apical localization of ENaC is inappropriately low in the sgk1-/- mice given the severalfold higher plasma aldosterone levels in the KO mice. Nevertheless, these data, together with the rather mild phenotype of sgk1-/- mice, as compared to the much more severe and life-threatening phenotypes of MR or ENaC KO mice, suggest that (a) aldosterone-dependent control of ENaC function does not solely rely on the induction and activation of SGK1 and (b) some redundancy exists in the signal transduction pathway that controls ENaC activity. Consistent with these ideas, Loffing and his coworkers found significant phosphorylation of the SGK1 target Nedd4-2 in mouse mpkCCDcl4 cells in vitro
[page 20] and in rat collecting ducts in vivo in the absence of any aldosterone and detectable SGK1 protein expression (Flores et al., 2005). In addition to aldosterone-dependent regulation of renal Na+ reabsorption, SGK1 appears to be involved also in the regulation of aldosterone-induced salt appetite. Sgk1+/+ and sgk1-/- mice show a similar salt intake under standard conditions. Treatment with the synthetic aldosterone analogue deoxycorticosterone-acetate (DOCA) increases Na+ intake much more in Sgk1+/+ mice than in sgk1-/- mice. The underlying mechanism for the reduced mineralocorticoid-induced salt intake is unclear (Vallon et al., 2005). 1.6 Role of SGK1 in Renal K+ Secretion Aside from its stimulatory effect on renal Na+ reabsorption, aldosterone has strong kaliuretic action. Renal K+ secretion also takes place in the ASDN and is likely mediated by the renal outer medullary K+ channel ROMK. ROMK is coexpressed with ENaC in the ASDN cells, and Na+ reabsorption via ENaC provides the necessary driving force for K+ secretion. Consistently, pharmacological inhibition (i.e., by amiloride) or genetic loss of function [i.e., pseudohypoaldosteronism (PHA) type 1] of ENaC lower renal K+ secretion and predispose one to hyperkalemia. It remains unresolved whether the kaliuretic effect of aldosterone is entirely secondary to the activation of ENaC-mediated Na+ reabsorption or whether aldosterone directly regulates ROMK function. |
The source is not given. |
|
| [35.] Dsa/Fragment 036 01 - Diskussion Bearbeitet: 6. August 2016, 10:38 WiseWoman Erstellt: 17. May 2015, 19:38 (Hindemith) | Boini 2006, Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 36, Zeilen: 1ff (entire page) |
Quelle: Boini 2006 Seite(n): 18, 19, Zeilen: 18: 8ff; 19: 1ff |
|---|---|
| At least part of the stimulatory effect of aldosterone on SGK1 appears to be mediated by activation of the MR, as indicated by findings in primary rabbit collecting duct cells in vitro (Narey-Fejes-Toth et al., (1999) [sic] J Biol Chem) and kidneys in vivo (Bhargava A. et al., (2001) Endocrinology). Consistently, physiologically relevant concentrations of aldosterone are sufficient to significantly induce SGK1 mRNA in the renal cortex and outer medulla (Muller OG. et al., (2003) J Am Soc Nephrol). The physiological importance of aldosterone in SGK induction is also supported by the fact that dietary Na+ restriction, which physiologically increases plasma aldosterone, induces SGK1 mRNA in the renal cortex (Farjah M. et al., (2003) Hypertension). The aldosterone-dependent induction of SGK1 occurs specifically in the ENaC-positive cells of the ASDN, whereas SGK1 expression in other nephron portions such as the thick ascending limb or the proximal tubule is not increased by aldosterone. Likewise, the high level of expression of SGK1 in the renal papilla is not further stimulated by aldosterone, suggesting that SGK1 expression at this site is controlled by factors other than aldosterone. The renal papilla plays an important role for the urinary concentration mechanism, and the cells in the renal papilla can be exposed to a large variation in extracellular osmolarity depending on the requirements for diuresis to antidiuresis. SGK1 expression is strongly modulated by osmotic cell shrinkage and swelling (Waldegger S. et al., (1997) Proc Natl Acad Sci USA; Rozansky DJ. et al., (2002) Am J Renal Physiol), and it is therefore conceivable that SGK1 participates in the functional adaptation of the renal papilla cells to fluctuation of extracellular osmolarity.
Consistent with this notion, recent data suggest that SGK1 mediates the osmotic induction of the type A natriuretic peptide receptor (NPR-A) in rat inner MCD cells (Chen S. et al., (2004) Hypertension). Aldosterone also controls SGK1 expression in the distal colon (Coric CM. et al., (2004) Am J Physiol Gastrointest Liver Physiol; Bhargava A. et al., (2001) Endocrinology). Aldosterone-dependent Na+ reabsorption at this site may help to limit extrarenal Na+ losses during conditions of dietary Na+ restriction. Transepithelial Na+ transport is achieved mainly by epithelial cells that are situated at the tips of colonic crypts and that express high levels of ENaC (Coric CM. et al., (2004) Am J Physiol Gastrointest Liver Physiol) and SGK1 (Waldegger S. et al., (1999) Gastroenterology; Coric CM. et al., (2004) Am J Physiol Gastrointest Liver Physiol). In spite of these data pointing to aldosterone-dependent regulation of ENaC via SGK1, recent Western blot and immunohistochemical studies on rat kidney and colon, which reported no or rather modest aldosterone-dependent induction of SGK1 at the protein level, were interpreted to question the significance of aldosterone-dependent induction of SGK1 for ENaC-mediated Na+ transport regulation (Coric CM. et al., (2004) Am J Physiol Gastrointest Liver Physiol). Support for a functional significance of SGK1 in regulation of transepithelial Na+ transport comes from experiments in X. laevis A6 cells and in mouse M1 CCD cells. Transfection of A6 or M1 cells with SGK1 leads to an increase in transepithelial Na+ transport, whereas transfection of a dominant-negative “kinase-dead” SGK1 mutant or an antisense SGK1 transcript abolishes dexamethasone- and/or insulin-dependent regulation of transepithelial Na+ transport (Alvarez de Rosa D. et al., (2003) J Physiol; Faletti CJ. et al., (2002) Am J Physiol Cell Physiol). Likewise, the use of interfering RNA to knockdown SGK1 expression in A6 cells results in a significant reduction in SGK1 protein levels and a ~50% reduction in dexamethasone-induced short-circuit currents (Bhargava A. et al., (2001) Endocrinology). Consistent with these in vitro findings, experiments in SGK1 KO (sgk1−/−) mice supported the importance of SGK1 for aldosterone-dependent regulation of renal Na+ transport (Wulff et al., (2002) J Clin Invest). |
At least part of the stimulatory effect of aldosterone on SGK1 appears to be mediated by activation of the MR, as indicated by findings in primary rabbit collecting duct cells in vitro (Naray-Fejes-Toth et al., 1999) and kidneys in vivo (Bhargava et al., 2001). Consistently, physiologically relevant concentrations of aldosterone are sufficient to significantly induce SGK1 mRNA in the renal cortex and outer medulla (Muller et al., 2003). The physiological importance of aldosterone in SGK induction is also supported by the fact that dietary Na+ restriction, which physiologically increases plasma aldosterone, induces SGK1 mRNA in the renal cortex (Farjah et al., 2003). The aldosterone-dependent induction of SGK1 occurs specifically in the ENaC-positive cells of the ASDN, whereas SGK1 expression in other nephron portions such as the thick ascending limb or the proximal tubule is not increased by aldosterone. Likewise, the high level of expression of SGK1 in the renal papilla is not further stimulated by aldosterone, suggesting that SGK1 expression at this site is controlled by factors other than aldosterone. The renal papilla plays an important role for the urinary concentration mechanism, and the cells in the renal papilla can be exposed to a large variation in extracellular osmolarity depending on the requirements for diuresis to antidiuresis. SGK1 expression is strongly modulated by osmotic cell shrinkage and swelling (Waldegger et al., 1997; Rozansky et al., 2002), and it is therefore conceivable that SGK1 participates in the functional adaptation of the renal papilla cells to fluctuation of extracellular osmolarity. Consistent with this notion, recent data suggest that SGK1 mediates the osmotic induction of the type A natriuretic peptide receptor (NPR-A) in rat inner MCD cells (Chen et al., 2004). Aldosterone also controls SGK1 expression in the distal colon (Coric et al., 2004; Bhargava et al., 2001). Aldosterone-dependent Na+ reabsorption at this site may help to limit extrarenal Na+ losses during conditions of dietary Na+ restriction. Transepithelial Na+ transport is achieved mainly by epithelial cells that are situated at the tips of colonic crypts and that express high levels of
[page 19] ENaC (Coric et al., 2004) and Sgk1 (Waldegger et al., 1999; Coric et al., 2004). In spite of these data pointing to aldosterone-dependent regulation of ENaC via SGK1, recent Western blot and immunohistochemical studies on rat kidney and colon, which reported no or rather modest aldosterone-dependent induction of SGK1 at the protein level, were interpreted to question the significance of aldosterone-dependent induction of SGK1 for ENaC-mediated Na+ transport regulation (Coric et al., 2004). Support for a functional significance of SGK1 in regulation of transepithelial Na+ transport comes from experiments in X. laevis A6 cells and in mouse M1 CCD cells. Transfection of A6 or M1 cells with SGK1 leads to an increase in transepithelial Na+ transport, whereas transfection of a dominant-negative “kinase-dead” SGK1 mutant or an antisense SGK1 transcript abolishes dexamethasone- and/or insulin-dependent regulation of transepithelial Na+ transport (Alvarez de Rosa et al., 2003; Faletti et al., 2002). Likewise, the use of interfering RNA to knockdown SGK1 expression in A6 cells results in a significant reduction in SGK1 protein levels and a ~50% reduction in dexamethasone-induced short-circuit currents (Bhargava et al., 2004). Consistent with these in vitro findings, experiments in SGK1 KO (sgk1-/-) mice supported the importance of SGK1 for aldosterone-dependent regulation of renal Na+ transport (Wulff et al., 2002). |
The source is not given. |
|
| [36.] Dsa/Fragment 035 01 - Diskussion Bearbeitet: 5. August 2016, 12:54 WiseWoman Erstellt: 17. May 2015, 19:34 (Hindemith) | Boini 2006, Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 35, Zeilen: 1ff (entire page) |
Quelle: Boini 2006 Seite(n): 16, 17, 18, Zeilen: 16: 17ff; 17: 1ff; 18: 1ff |
|---|---|
| Experiments in heterologous expression systems (i.e., X. laevis oocytes) revealed that coexpression of either ENaC or Na+ (consensus: R-S-X-pS-X-P) increases SGK stimulation. Indeed, in X. laevis oocytes, SGK1 increases the binding of 14-3-3 to Nedd4-2 in a phosphorylation-dependent manner, a dominant-negative 14-3-3 mutant profoundly attenuates SGK1-dependent stimulation of ENaC, and overexpression of the 14-3-3 protein impairs Nedd4-2-dependent ubiquitylation of ENaC (Ichimura T. et al., (2005) J Biol Chem).
In addition to this indirect action of SGK1 on ENaC cell surface abundance, it was proposed that SGK1 can directly interact with ENaC (Wang J. et al., (2001) Am J Physiol Renal Physiol) and increase ENaC channel activity by phosphorylating the α-ENaC subunits (Diakov A. and Korbmacher C., (2004) J Biol Chem). Diakov and Korbmacher used outsideout membrane patches of X. laevis oocytes expressing rat ENaC to demonstrate that addition of recombinant, constitutively active SGK1 directly stimulates ENaC currents two- to threefold. An alanine mutation of the serine residue in the SGK1 consensus R-X-R-X-X-S phosphorylation motif abolishes the stimulatory effect on ENaC in this experimental setting. Experiments in native Xenopus A6 cells expressing endogenous SGK1 and ENaC further confirmed that the action of SGK1 on ENaC is complex and likely involves (a) increases in the subunit abundance in the plasma membrane and (b) activation of channels already in the plasma membrane combined with an increase in ENaC open probability (Alvarez de la Rosa D. et al., (2004) J Physiol). However, in this model the stimulatory effect on ENaC channel activity cannot be explained by a direct SGK1-dependent phosphorylation of α-ENaC because Xenopus α-ENaC does not contain the SGK1 consensus phosphorylation motif. That direct phosphorylation of ENaC at the SGK1 consensus site is not essential for ENaC activation is also supported by data from Lang and coworkers (Lang F. et al., (2000) Proc Natl Acad Sci USA; Friedrich B. et al., (2002) Kidney Blood Press Res) that showed that channels with a serine-to-alanine mutation within the consensus site of α-ENaC are still rigorously upregulated by coexpression of SGK1 in Xenopus oocytes. NDRG-2, which is an aldosterone-induced protein in the ASDN, is another target of SGK1 (Boulkroum S. et al., (2002) J Biol Chem; Murray JT. et al., (2004) Biochem J). Although the functional role of NDRG-2 in the ASDN is not known, this protein may also have some function in the SGK1-dependent signaling cascade related to Na+ transport. As an aldosterone-induced protein, SGK1 is thought to mediate at least some of the physiological effects of aldosterone on ENaC and Na+, K+-ATPase. The stimulatory effect of aldosterone (or of dexamethasone) on SGK1 expression has now been firmly documented in several studies on various in vitro and in vivo systems, including Xenopus A6 cells (Bhargava A. et al., (2004) Trends Endocrinol Metab), primary rabbit CCD cells (Narey-Fejes-Toth et al., (1999) J BiolChem), mouse inner MCD cells (Gumz et al., (2003) Am J Physiol Renal Physiol), mouse mpkCCDcl4 (Flores SY. et al., (2005) J Am Soc Nephrol), mouse M1 cells (Helms MN. et al., (2003) Am J Physiol Renal Phyisol) and mouse and rat kidneys (Chen et al., (1999) Proc Natl Acad Sci USA; Loffing et al.,(2001) Am J Physiol Renal Physiol; Bhargava A. et al., (2001) Endocrinology). Corticosteroids rapidly (within 30 minutes) induce SGK1 at the mRNA and/or protein levels. This induction precedes or at least coincides with enhanced phosphorylation of Nedd4-2 (Flores SY. et al., (2005) J Am Soc Nephrol), the activation of transepithelial Na+ transport in cultured renal epithelia (Naray-Fejes-Toth A. et al., (1999) J Biol Chem; Bhargava A. et al., (2004) Trends Endocrinol Metab; Flores SY. et al., (2005) J Am Soc Nephrol), and reduced renal Na+ secretion in intact animals (Bhargava A. et al., (2001) Endocrinology). |
Experiments in heterologous expression systems (i.e., X. laevis oocytes) revealed that coexpression of either ENaC or Na+, [...]
[page 17] (consensus: R-S-X-pS-X-P). Indeed, in X. laevis oocytes, SGK1 increases the binding of 14-3-3 to Nedd4-2 in a phosphorylation-dependent manner, a dominant-negative 14-3-3 mutant profoundly attenuates SGK1-dependent stimulation of ENaC, and overexpression of the 14-3-3 protein impairs Nedd4-2-dependent ubiquitylation of ENaC (Ichimura et al., 2005). In addition to this indirect action of SGK1 on ENaC cell surface abundance, it was proposed that SGK1 can directly interact with ENaC (Wang et al., 2001) and increase ENaC channel activity by phosphorylating the α-ENaC subunits (Diakov and Korbmacher 2004). Diakov & Korbmacher (2004) used outside-out membrane patches of X. laevis oocytes expressing rat ENaC to demonstrate that addition of recombinant, constitutively active SGK1 directly stimulates ENaC currents two- to threefold. An alanine mutation of the serine residue in the SGK1 consensus R-X-R-X-X-S phosphorylation motif abolishes the stimulatory effect on ENaC in this experimental setting. Experiments in native Xenopus A6 cells expressing endogenous SGK1 and ENaC further confirmed that the action of SGK1 on ENaC is complex and likely involves (a) increases in the subunit abundance in the plasma membrane and (b) activation of channels already in the plasma membrane combined with an increase in ENaC open probability (Alvarez de la Rosa et al., 2004). However, in this model the stimulatory effect on ENaC channel activity cannot be explained by a direct SGK1-dependent phosphorylation of α-ENaC because Xenopus α-ENaC does not contain the SGK1 consensus phosphorylation motif. That direct phosphorylation of ENaC at the SGK1 consensus site is not essential for ENaC activation is also supported by data from Lang and coworkers (Lang et al., 2000; Friedrich et al., 2002) that showed that channels with a serine-to-alanine mutation within the consensus site of α-ENaC are still rigorously upregulated by coexpression of SGK1 in Xenopus oocytes. NDRG-2, which is an aldosterone-induced protein in the ASDN, is another target of SGK1 (Boulkroum et al., 2002; Murray et al., 2004). Although the functional role of NDRG-2 in the ASDN is not known, this protein may also have some function in the SGK1-dependent signaling cascade related to Na+ transport. As an aldosterone-induced protein, SGK1 is thought to mediate at least some of the physiological effects of aldosterone on ENaC and Na+,K+-ATPase. The stimulatory effect of aldosterone (or of dexamethasone) on SGK1 expression has now been firmly documented in several studies on various in vitro and in vivo systems, including Xenopus A6 cells (Bhargava et al., 20004), primary rabbit CCD cells (Narey-Fejes-Toth et [page 18] al., 1999), mouse inner MCD cells (Gumz et al., 2003), mouse mpkCCDcl4 (Flores et al., 2005), mouse M1 cells (Helms et al., 2003 ), and mouse and rat kidneys (Chen et al., 1999; Loffing et al., 2001; Bhargava et al., 2001). Corticosteroids rapidly (within 30 minutes) induce SGK1 at the mRNA and/or protein levels. This induction precedes or at least coincides with enhanced phosphorylation of Nedd4-2 (Flores et al., 2005), the activation of transepithelial Na+ transport in cultured renal epithelia (Naray-Fejes-Toth et al., 1999; Bhargava et al., 2004; Flores et al., 2005), and reduced renal Na+ secretion in intact animals (Bhargava et al., 2001). |
The source is not given. |
|
| [37.] Dsa/Fragment 034 01 - Diskussion Bearbeitet: 4. August 2016, 22:00 WiseWoman Erstellt: 17. May 2015, 19:27 (Hindemith) | Boini 2006, Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 34, Zeilen: 1-20 |
Quelle: Boini 2006 Seite(n): 16, Zeilen: 1ff |
|---|---|
| This inhibitory effect of SGK1 on Nedd4-2 likely involves 14-3-3 proteins as phosphorylation of Ser444 in Nedd4-2 creates a possible binding site for such proteins, an inherited form of salt-sensitive hypertension are caused by mutations in the genes encoding β- and γ -ENaC (Hansson JH. et al., (1995) Proc Natl Acad Sci USA; Shimkets RA. et al., (1994) Cell). These mutations invariably cause either the deletion or the mutation of the PY-motifs on these subunits. When such Liddle channels are expressed in heterologous systems, increases in both the density at the cell surface and the open probability of ENaC are observed (Firsov et al., (1996) Proc Natl Acad Sci USA; Snyder PM. et al., (1994) Cell; Schild et al., (1995) Proc Natl Acad Sci USA; Schild et al., (1996) EMBO J). Loffing and his coworkers, has demonstrated that these PY-motifs are the binding sites for ubiquitin-protein ligases of the Nedd4/Nedd4-like family (Kamynina E. et al., (2001) EMBO J) and particularly of Nedd4-2 (Kamynina E. et al., (2001) EMBO J; Snyder PM. et al., (2004) J Biol Chem).
It is thought that Nedd4-2 binds via its WW domains with the PY-motifs of ENaC and ubiquitylates ENaC on its own α and γ subunits, consequently leading to the internalization and degradation of ENaC in the endosomal/lysosomal system (Snyder PM. et al., (2004) J Biol Chem). In Liddle’s syndrome, this mechanism is impaired owing to the inactivation of a PY-motif, causing the accumulation of ENaC at the plasma membrane (Kamynina E. and Staub O., (2002) Am J Physiol Renal Physiol). The activity of ENaC and the Na+, K+-ATPase is tightly regulated by aldosterone and by SGK1 (Vallon V. et al., (2005) Am J Physiol Regul Integr Comp Physiol; Bhargava A. et al., (2004) Trends Endocrinol Metab). |
[...], an inherited form of salt-sensitive hypertension are caused by mutations in the genes encoding β- and γ -ENaC (Hansson et al., 1995; Shimkets et al., 1994). These mutations invariably cause either the deletion or the mutation of the PY-motifs on these subunits. When such Liddle channels are expressed in heterologous systems, increases in both the density at the cell surface and the open probability of ENaC are observed (Firsov et al., 1996; Snyder et al., 1995; Schild et al., 1995; Schild et al., 1996). Loffing and his coworkers, has demonstrated that these PY-motifs are the binding sites for ubiquitin-protein ligases of the Nedd4/Nedd4-like family (Kamynina et al., 2001; Kamynina et al., 2001a ) and particularly of Nedd4-2 (Kamynina et al., 2001; Kamynina et al., 2001a; Snyder et al., 2004). It is thought that Nedd4-2 binds via its WW domains with the PY-motifs of ENaC and ubiquitylates ENaC on its α and γ subunits, consequently leading to the internalization and degradation of ENaC in the endosomal/lysosomal system (Snyder et al., 2004). In Liddle’s syndrome, this mechanism is impaired owing to the inactivation of a PY-motif, causing the accumulation of ENaC at the plasma membrane (Kamynina and Staub 2002). The activity of ENaC and the Na+, K+-ATPase is tightly regulated by aldosterone and by SGK1 (Kellenberger and Schild 2002; Vallon et al., 2005; Bhargava et al., 2004). [...] This inhibitory effect of SGK1 on Nedd4-2 likely involves 14-3-3 proteins as phosphorylation of Ser444 in Nedd4-2 creates a possible binding site for such proteins |
The source is not given. Note that the first sentence makes little sense, as it is a combination of two unrelated half-sentences of the source. Apparently the last line of the source on page 16 has not been continued with the first line on page 17, but with the first line on page 16 again, leading to this result. Also, the source writes Ser444, Dsa writes Ser444 constistently. |
|
| [38.] Dsa/Fragment 032 01 - Diskussion Bearbeitet: 4. August 2016, 21:51 WiseWoman Erstellt: 17. May 2015, 19:11 (Hindemith) | Boini 2006, Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 32, Zeilen: 1-22 |
Quelle: Boini 2006 Seite(n): 15, 16, Zeilen: 15: 16ff; 16: 18ff |
|---|---|
| Transepithelial Na+ transport in these segments is accomplished by Na+ entry into the epithelial cells via the epithelial Na+ channel (ENaC) in the luminal membrane and by exit of Na+ through the Na+, K+-ATPase in the basolateral plasma membrane. ENaC represents the rate limiting step in this process and is highly regulated (Kellenberger S. and Schild L., (2002) J Physiol). It is composed of three subunits (α, β and γ) (Canessa CM. et al., (1994) Nature; Lingueglia et al., (1993) FEBS Lett; Lingueglia et al., (1994) J Biol Chem) with a stoichiometry of 2α1β1γ (Firsov et al., (1998) EMBO J), although other stoichiometries have also been proposed (octa- or nonamers) (Eskandari et al., (1999) J Biol Chem; Snyder et al., (1998) J Biol Chem). Its subunits have a similar topology, with two transmembrane domains, one extracellular loop, and two cytoplasmic ends (Renard et al., (1994) J Biol Chem; Canessa CM. et al., (1994) Nature; Snyder et al., (1994) PMID). Each subunit also contains, at its C-terminal end, a PY-motif (P-P-X-Y, where P is a proline, Y a tyrosine, and X any amino acid), which is known as protein: protein interaction motifs that can interact with tryptophan (W)-rich WW domains (Chen HI. and Sudol M., (1995) Proc Natl Acad Sci USA; Staub O. and Rotin D., (1996) Am Physiol Soc).
The importance of these PY-motifs for ENaC regulation has been recognized by the findings that most cases of Liddle’s syndrome, K+-ATPase (Zecevic M. et al., (2004) Pflügers Arch; Setiawan I. et al., (2002) Pflügers Arch) with SGK1 profoundly increases the activity of both Na+- transporting proteins. Likewise, SGK2 and SGK3 stimulate ENaC (Friedrich B. et al., (2003) Pflügers Arch) and Na+- K+-ATPase (Henke G. et al., (2002) Kidney Blood Press Res). |
Transepithelial Na+ transport in these segments is accomplished by Na+ entry into the epithelial cells via the epithelial Na+ channel (ENaC) in the luminal membrane and by exit of Na+ through the Na+, K+-ATPase in the basolateral plasma membrane. ENaC represents the rate limiting step in this process and is highly regulated (Kellenberger and Schild 2002). It is composed of three subunits (α, β and γ) (Canessa et al., 1994; Canessa et al., 1994; Lingueglia et al., 1993; Lingueglia et al., 1994) with a stoichiometry of 2α1β1γ (Firsov et al., 1998), although other stoichiometries have also been proposed (octa- or nonamers) (Eskandari et al., 1999; Snyder et al., 1998). Its subunits have a similar topology, with two transmembrane domains, one extracellular loop, and two cytoplasmic ends (Renard et al., 1994; Canessa et al., 1994; Snyder et al., 1994). Each subunit also contains, at its C-terminal end, a PY-motif (P-P-X-Y, where P is a proline, Y a tyrosine, and X any amino acid), which is known as protein:protein interaction motifs that can interact with tryptophan (W)-rich WW domains (Chen and Sudol 1995; Staub and Rotin 1996). The importance of these PY-motifs for ENaC regulation has been recognized by the findings that most cases of Liddle’s syndrome [(Liddle et al., 1963)]
[p. 16]
|
The source is not mentioned. Note that the first sentence of the second paragraph makes little sense, as it is a combination of two unrelated half-sentences of the source, the first one ending just at the page break from page 15 to page 16. |
|
| [39.] Dsa/Fragment 031 34 - Diskussion Bearbeitet: 3. August 2016, 20:12 WiseWoman Erstellt: 17. May 2015, 19:06 (Hindemith) | Boini 2006, Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 31, Zeilen: 34-42 |
Quelle: Boini 2006 Seite(n): 15, Zeilen: 8ff |
|---|---|
| a) Role of SGK1 in aldosterone dependent Na+ reabsorption
Although SGK isoforms are expressed in various tissues and cell types, the role of SGK1 in aldosterone-dependent regulation of Na+ homeostasis is the best-studied function of these kinases with respect to epithelial ion transport. The kidneys play a pivotal role in the maintenance of Na+ homeostasis. Urinary Na+ excretion must be tightly regulated to maintain a constant extracellular volume during varying dietary Na+ intake and extrarenal Na+ losses. The final control of renal Na+ excretion is achieved by the ASDN i.e. the late distal convoluted tubule, the connecting tubule and the cortical as well as the medullary collecting ducts (CCD and MCD respectively) (Loffing J. et al., (2001) Am J Physiol Renal Physiol). |
1.5 Role of SGK1 in Aldosterone Dependent Na+ Reabsorption
Although SGK isoforms are expressed in various tissues and cell types, the role of SGK1 in aldosterone-dependent regulation of Na+ homeostasis is the best-studied function of these kinases with respect to epithelial ion transport. The kidneys play a pivotal role in the maintenance of Na+ homeostasis. Urinary Na+ excretion must be tightly regulated to maintain a constant extracellular volume during varying dietary Na+ intake and extrarenal Na+ losses. The final control of renal Na+ excretion is achieved by the ASDN i.e. the late distal convoluted tubule, the connecting tubule, and the cortical as well as the medullary collecting ducts (CCD and MCD respectively) (Loffing et al., 2001). |
The source is not given. |
|
| [40.] Dsa/Fragment 028 01 - Diskussion Bearbeitet: 3. August 2016, 19:01 WiseWoman Erstellt: 17. May 2015, 19:04 (Hindemith) | Boini 2006, Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 28, Zeilen: 1-45 |
Quelle: Boini 2006 Seite(n): 12, 14, 15, Zeilen: 12: 11ff; 14: 12ff; 15: 1ff |
|---|---|
| [Like SGK2, SGK3 has been implicated in the regulation of numerous] transporters and channels, including K+ channels (Gamper et al., (2002) Pflügers Arch; Henke et al., (2004) J cell Physiol); Embark et al., (2003) Pflügers Arch), Na+-K+-ATPase (Henke G. et al., (2002) Kidney Blood Press Res), the glutamate transporter EEAT1 (Boehmer et al., (2003) Cardiovasc Res), the cardiac voltage-gated Na+ channel SCN5A (Boehmer et al., (2003) J Neurochem), ENaC (Friedrich et al., (2003) Pflügers Arch), Na+-dicarboxylate cotransporter 1 (Böhmer et al., (2004) Biochem Biophys Res Commun), the chloride channel ClCa/barttin (Embark et al., (2004) Pflügers Arch), the epithelial Ca2+ channel TRPV5 (Embark et al., (2004) Pflügers Arch), the Na+-phosphate cotransporter NaPi2b (Palmada M. et al., (2004) Cell Physiol Biochem), the amino acid transporter ASCT2 (Palmada M. et al., (2005) Cell Physiol Biochem), GluR1, and GluR6 (Strutz-Seebohm et al., (2005); Strutz-Seebohm et al., (2005a) J Physiol). For the same reasons mentioned above for SGK2, additional studies on SGK3 will be necessary to evaluate the physiological relevance of these findings.
1.4. Tissue distribution of SGK isoforms SGK isoforms are expressed in numerous tissues and cell lines. Among the three kinases, SGK1 (Waldegger et al., (1997) Proc Natl Acad Sci USA; Kobayashi et al., (1999) Biochem J) and SGK3 show the broadest distribution, with expression in many tissues including the brain, placenta, lung, liver, pancreas, kidney, heart and skeletal muscle. In situ hybridization studies localized SGK1 mRNA in several epithelial and/or nonepithelial cells within the brain (Wärntges et al., (2002) Cell Physiol Biochem; Nishida et al., (2004) Brain Res; Tsai et al., (2002) Proc Natl Acad Sci USA; Stichel et al., (2005) Eur J Neurosci; Gonzalez-Nicolini A. and McGinty F., (2002) Brain Res Gene ExpPatterns), eye (Rauz et al., (2003) Exp Eye Res; Rauz et al., (2003a) Invest Ophtalmol Vis Sci), lung (Wärntges et al., (2002) Cell Physiol Biochem) liver (Fillon et al., (2002) Comp Biochem Physiol A Mol Integr Physiol), ovary (Alliston et al., (2000) Endocrinology), pancreas (Klingel et al., (2000) Am J Physiol Gastrointest Liver Physiol), intestine (Waldegger et al., (1999) Gastroentherology) and kidney (Chen et al., (1999) Proc Natl Acad Sci USA; Friedrich et al., (2002) Kidney Blood Press res; Huber et al., (2001) Pflügers Archive). SGK1 mRNA expression is established very early in embryonic development, as indicated by in situ hybridizations on whole-mount preparations of mouse embryo (Lee et al., (2001) Mech Dev). By embryonic day (E) 8.5, SGK1 is already highly expressed in the decidua and yolk sac. By days E9.5– E12.5 it is found in the developing heart, eye, and lung, and it becomes highly expressed by days E13.5–E16.5 in the brain choroid plexus, kidney distal tubules, bronchi/bronchiole, adrenal glands, liver, thymus, and intestine (Lee et al., (2001) Mech Dev). In contrast to SGK1 and SGK3, SGK2 reveals a more restricted distribution and is highly abundant only in the liver, kidney, and pancreas, where it is found in two different SGK2 species, referred to as SGK2α and SGK2β (Kobayashi et al., (1999) Biochem J). SGK isoform expression varies also between cell lines cultured in vitro. Similar to its expression pattern in vivo, SGK1 is broadly expressed in cultured cells and is readily detectable in, for example, hepatoma cells, fibroblasts and mammary tumor cells (Webster et al., (1993) J Biol Chem; Kobayashi et al., (1999) Biochem J). By contrast, SGK2 mRNA is expressed in hepatoma cells but not in fibroblasts, whereas SGK3 is found in fibroblasts but not in hepatoma cells. Remarkably, all three SGK isoforms are expressed in cells derived from the renal cortical collecting duct (Naray-Fejes-Toth et al., (2004) Proc Natl Acad Sci USA). |
[p. 12]
Like SGK2, SGK3 has been implicated in the regulation of numerous transporters and channels, including K+ channels (Gamper et al., 2002; Henke et al., 2004; Embark et al., 2003), Na+,K+-ATPase (Henke et al., 2002), the glutamate transporter EEAT1 (Boehmer et al., 2003), the cardiac voltage-gated Na+ channel SCN5A (Boehmer et al., 2003), ENaC (Friedrich et al., 2003), Na+-dicarboxylate cotransporter 1 (Boehmer et al., 2004), the chloride channel ClCa/barttin (Embark et al., 2004), the epithelial Ca2+ channel TRPV5 (Embark et al., 2004), the Na+-phosphate cotransporter NaPi1b (Palmada et al., 2004), the amino acid transporter ASCT2 (Palmada et al., 2005), GluR1, and GluR6 (Strutz-Seebohm et al., 2005; Strutz-Seebohm et al., 2005a). For the same reasons mentioned above for SGK2, additional studies on SGK3 will be necessary to evaluate the physiological relevance of these findings. [...] [p. 14] 1.4 Tissue Distribution of SGK Isoforms SGK isoforms are expressed in numerous tissues and cell lines. Among the three kinases, SGK1 (Webster et al., 1993; Waldegger et al., 1997; Kobayashi et al., 1999) and Sgk3 (Kobayashi et al., 1999) show the broadest distribution, with expression in many tissues including the brain, placenta, lung, liver, pancreas, kidney, heart and skeletal muscle. In situ hybridization studies localized SGK1 mRNA in several epithelial and/or nonepithelial cells within the brain (Warntges et al., 2002; Nishida et al., 2004; Tsai et al., 2002; Stichel et al., 2005; Gonzalez-Nicolini and McGinty 2003), eye (Rauz et al., 2003; Rauz et al., 2003a), lung (Waerntges et al., 2002), liver (Fillon et al., 2002), ovary (Alliston et al., 2000), pancreas (Klingel et al., 2000), intestine (Waldegger et al., 1999), and kidney (Chen et al., 1999; Friedrich et al., 2002; Huber et al., 2001). SGK1 mRNA expression is established very early in embryonic development, as indicated by in situ hybridizations on whole-mount preparations of mouse embryo (Lee et al., 2001). By embryonic day (E) 8.5, SGK1 is already highly expressed in the decidua and yolk sac. By days E9.5– E12.5 it is found in the developing heart, eye, and lung, and it becomes highly expressed by days E13.5–E16.5 in the brain choroid plexus, kidney distal tubules, bronchi/brochioli, adrenal glands, liver, thymus, and intestine (Lee et al., 2001). In contrast to SGK1 and SGK3, SGK2 reveals a more restricted distribution and is highly abundant only in the liver, kidney, and pancreas, where it is found in two different SGK2 species, referred to as SGK2α and [p. 15] SGK2β (Kobayashi et al., 1999). SGK isoform expression varies also between cell lines cultured in vitro. Similar to its expression pattern in vivo, SGK1 is broadly expressed in cultured cells and is readily detectable in, for example, hepatoma cells, fibroblasts, and mammary tumor cells (Webster et al., 1993; Kobayashi et al., 1999). By contrast, SGK2 mRNA is expressed in hepatoma cells but not in fibroblasts, whereas SGK3 is found in fibroblasts but not in hepatoma cells. Remarkably, all three SGK isoforms are expressed in cells derived from the renal cortical collecting duct (Naray-Fejes-Toth et al., 2004). |
The source is not given. |
|
| [41.] Dsa/Fragment 026 10 - Diskussion Bearbeitet: 3. August 2016, 17:29 WiseWoman Erstellt: 18. May 2015, 10:02 (Hindemith) | Boini 2006, Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 26, Zeilen: 10-13 |
Quelle: Boini 2006 Seite(n): 10, Zeilen: 1ff |
|---|---|
| 1.3.2. The SGK1 Isoforms SGK2 and SGK3
There are two paralogs of SGK1, SGK2 and SGK3/CISK, which share 80% amino acid identity with SGK1 and with each other in their catalytic domains (Kobayashi T. et al., (1999) Biochem J; Liu D. et al., (2000) Curr Biol; Dai F. et al., (1999) Genomics). |
1.2 The SGK1 Isoforms SGK2 and SGK3
There are two paralogs of SGK1, SGK2 and SGK3/CISK, which share 80% amino acid identity with SGK1 and with each other in their catalytic domains (Kobayashi et al., 1999; Liu et al., 2000; Dai et al., 1999). |
The source is not given here. |
|
| [42.] Dsa/Fragment 033 01 - Diskussion Bearbeitet: 3. August 2016, 17:18 WiseWoman Erstellt: 17. May 2015, 19:20 (Hindemith) | Boini 2006, Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 33, Zeilen: 1-13 |
Quelle: Boini 2006 Seite(n): 16, Zeilen: 21ff |
|---|---|
| The stimulatory effect of SGK1 on ENaC is related both to an increased number of channels in the plasma membrane (Lang F. et al., (2000) Proc Natl Acad Sci USA; Loffing J. et al., (2001) Am J Physiol Renal Physiol; Alvarez de la Rosa D. et al., (1999) J Biol Chem) and an activation of channels already present in the membrane (Diakov A. and Korbmacher C., (2004) J Biol Chem). The first effect likely involves the action of Nedd4-2, as there are several consensus phosphorylation motifs (2–3 depending on the splice variant) on Nedd4-2 and a PY-motif on SGK1 that may serve as a binding site for Nedd4-2.
In Xenopus oocytes, SGK1 induces Nedd4-2 phosphorylation on two of these phosphorylation sites (primarily Ser444, but also Ser338) (Embark HM. et al., (2004) Cell Physiol Biochem; Palmada M. et al., (2004) Am J Physiol Gastrointest Liver Physiol; Debonneville C. et al., (2001) EMBO J), which decreases the interaction of Nedd4-2 with ENaC and finally leads to an enhanced expression and activity of ENaC at the cell surface (Debonneville C. et al., (2001) EMBO J). |
The stimulatory effect of SGK1 on ENaC is related both to an increased number of channels in the plasma membrane (Lang et al., 2000; Loffing et al., 2001; Alvarez de la Rosa et al., 1999) and an activation of channels already present in the membrane (Diakov and Korbmacher 2004). The first effect likely involves the action of Nedd4-2, as there are several consensus phosphorylation motifs (2–3 depending on the splice variant) on Nedd4-2 and a PY-motif on SGK1 that may serve as a binding site for Nedd4-2. In Xenopus oocytes, SGK1 induces Nedd4-2 phosphorylation on two of these phosphorylation sites (primarily Ser444, but also Ser338) (Embark et al., 2004; Palmada et al., 2004; Debonneville et al., 2001), which decreases the interaction of Nedd4-2 with ENaC and finally leads to an enhanced expression and activity of ENaC at the cell surface (Debonneville et al., 2001). |
The source is not given. |
|
| [43.] Dsa/Fragment 027 04 - Diskussion Bearbeitet: 3. August 2016, 17:13 WiseWoman Erstellt: 17. May 2015, 18:59 (Hindemith) | Boini 2006, Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 27, Zeilen: 4-50 |
Quelle: Boini 2006 Seite(n): 10, 11, 12, Zeilen: 10: 4ff; 11: 1ff; 12: 1ff |
|---|---|
| The three enzymes differ in the region N-terminal of the C-terminal catalytic domain: SGK2 contains a relatively short N terminus (98 amino acids), with no discernable domain, whereas SGK3 has a longer N terminus (162 amino acids) comprising a phox homology (PX) domain (Xu J. et al., (2001) Cell Biol).
PX domains were originally found as conserved domains in the p40phox and p47phox subunits of the neutrophil NADPH oxidase (phox) superoxide-generating complex (Ponting CP., (1996) Protein Sci). These domains are part of many proteins involved in intracellular protein trafficking, such as the sorting nexins. PX domains are phosphoinositide-binding domains that appear to be important for localization of these proteins to membranes (especially endosomes) enriched in phosphoinositides. In this respect, these domains resemble other domains such as the pleckstrin homology (PH), FYFE, FERM and ENTH domains. PKB/Akt contains a PH domain in its N terminus, which is important for PKB/Akt activation by phosphoinositide-3 kinases (PI-3Ks). This domain enables the colocalization with the 3- phosphoinositide-dependent protein kinase-1 (PDK1), which is known to phosphorylate and activate PKB/Akt. Similarly, SGK3’s PX domain is involved in SGK3 localization and activity: It is necessary for phosphoinositide binding, endosomal localization, and proper kinase activity. Moreover, structural studies indicate that it may play a role in dimerization of the kinase. With respect to their physiological role(s), it has been shown in vitro that both the SGK2 and SGK3 enzymes have the same phosphorylation consensus as SGK1 (and PKB/Akt), namely R-X-R-X-X-(S/T). It is likely, however, that other factors, such as surrounding amino acids, subcellular localization, or cofactors are important for the specificity of and functional differences between the enzymes. For example, in Xenopus A6 cells, only SGK1 and not the coexpressed PKB modulates the activity of the epithelial Na+ channel (ENaC) (Arteaga MF. et al., (2005) Am J Physiol Renal Physiol). The role of SGK2 has mainly been studied in heterologous expression systems such as Xenopus laevis oocytes or HEK293 cells and with respect to numerous transport and channel proteins. These studies revealed that SGK2 can stimulate the activity of K+ channels such as the voltage- gated K+ channel Kv1.3 (Gamper et al., (2002) Pflügers Arc,; Henke et al., (2004) J Cell Physiol), Na+-K+-ATPase (Henke G. et al., (2002) Kidney Blood Press Res), KCNE1 (Embark et al., (2003) Pflügers Arch), ENaC (Friedrich et al., (2003) Pflügers Arch), the glutamate transporter EEAT4 (Böhmer et al., (2004) Biochem Biophys Res Commun), and the glutamate receptors GluR6 (Strutz-Seebohm et al., (2005) J Physiol) and GluR1 (Strutz-Seebohm et al., (2005a) J Physiol). All of these transport proteins are also stimulated in the same cellular systems by SGK1, SGK3, and/or PKB; hence, the physiological relevance of these findings has to be considered with caution. To define more precisely the role of SGK2, it will be necessary to carry out additional studies, using more relevant cell or animal systems and knocking down SGK2 by either RNA interference protocols or by gene inactivation. SGK3/CISK, which is better characterized than SGK2, was identified in a screen for antiapoptotic genes (Liu et al., (2000) Curr Biol) and found to act downstream of the PI-3K pathway and in parallel with PKB/Akt. Moreover, it was demonstrated to phosphorylate and inhibit Bad (a proapoptotic protein) and FKHRL1, a proapoptotic transcription factor. Knockout (ko) mice have been generated; these mice are viable and fertile and have normal Na+ handling and glucose tolerance, as opposed to the KO mice of SGK1 or PKB/Akt2 (McCormick et al., (2004) Mol Biol Cell; Garofalo et al., (2003) J Clin Invest; Wulff et al., (2002) J Clin Invest). However, they display after birth a defect in hair follicle development, a defect preceded by disturbances in the β-catenin/Lef1 gene regulation (McCormick et al., (2004) Mol Biol Cell). |
The three enzymes differ in the region N-terminal of the C-terminal catalytic domain: SGK2 contains a relatively short N terminus (98 amino
[page 11] acids), with no discernable domain, whereas SGK3 has a longer N terminus (162 amino acids) comprising a phox homology (PX) domain (Xu et al., 2001). PX domains were originally found as conserved domains in the p40phox and p47phox subunits of the neutrophil NADPH oxidase (phox) superoxide-generating complex (Ponting 1996). These domains are part of many proteins involved in intracellular protein trafficking, such as the sorting nexins (Worby and Dixon 2002). PX domains are phosphoinositide-binding domains that appear to be important for localization of these proteins to membranes (especially endosomes) enriched in phosphoinositides. In this respect, these domains resemble other domains such as the pleckstrin homology (PH), FYFE, FERM and ENTH domains. PKB/Akt contains a PH domain in its N terminus, which is important for PKB/Akt activation by phosphoinositide-3 kinases (PI-3Ks). This domain enables the colocalization with the 3-phosphoinositide-dependent protein kinase-1 (PDK-1), which is known to phosphorylate and activate PKB/Akt. Similarly, SGK3’s PX domain is involved in SGK3 localization and activity: It is necessary for phosphoinositide binding, endosomal localization, and proper kinase activity (Xu et al., 2001; Liu et al., 2000). Moreover, structural studies indicate that it may play a role in dimerization of the kinase (Xing et al., 2004). With respect to their physiological role(s), it has been shown in vitro that both the SGK2 and SGK3 enzymes have the same phosphorylation consensus as SGK1 (and PKB/Akt), namely R-X-R-X-X-(S/T) (Kobayashi et al., 1999). It is likely, however, that other factors, such as surrounding amino acids, subcellular localization, or cofactors are important for the specificity of and functional differences between the enzymes. For example, in Xenopus A6 cells, only SGK1 and not the coexpressed PKB modulates the activity of the epithelial Na+ channel (ENaC) (Artega et al., 2005). The role of SGK2 has mainly been studied in heterologous expression systems such as Xenopus laevis oocytes or HEK293 cells and with respect to numerous transport and channel proteins. These studies revealed that SGK2 can stimulate the activity of K+ channels such as the voltage- gated K+ channel Kv1.3 (Gamper et al., 2002; Henke et al., 2004), Na+,K+-ATPase (Henke et al., 2002), KCNE1 (Embark et al., 2003), ENaC (Friedrich et al., 2003), the glutamate transporter EEAT4 (Bohmer et al., 2004), and the glutamate receptors GluR6 (Strutz-Seebohm et al., 2005) and GluR1 (Strutz-Seebohm et al., 2005a). All of these transport proteins are also stimulated in the same cellular systems by SGK1, SGK3, and/or PKB; hence, the physiological relevance of these findings has to be considered with caution. To define more precisely the role of SGK2, it will be necessary to [page 12] carry out additional studies, using more relevant cell or animal systems and knocking down SGK2 by either RNA interference protocols or by gene inactivation. SGK3/CISK, which is better characterized than SGK2, was identified in a screen for antiapoptotic genes (Liu et al., 2000) and found to act downstream of the PI-3K pathway and in parallel with PKB/Akt. Moreover, it was demonstrated to phosphorylate and inhibit Bad (a proapoptotic protein) and FKHRL1, a proapoptotic transcription factor. Knockout (KO) mice have been generated; these mice are viable and fertile and have normal Na+ handling and glucose tolerance, as opposed to the KO mice of SGK1 or PKB/Akt2 (McCormick et al., 2004; Garofalo et al., 2003; Wulff et al., 2002; Cho et al., 2001). However, they display after birth a defect in hair follicle development, a defect preceded by disturbances in the β-catenein/Lef1 gene regulation (McCormick et al., 2004). |
The source is not given. |
|
| [44.] Dsa/Fragment 017 16 - Diskussion Bearbeitet: 2. August 2016, 19:33 WiseWoman Erstellt: 17. May 2015, 18:15 (Hindemith) | Dsa, Fragment, Gesichtet, Kinexus 2006, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 17, Zeilen: 16-23, 25-45 |
Quelle: Kinexus 2006 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| Approximately fifty of the hundred or so known genes that have been directly linked to induction of cancer (i.e. oncogenes) encode protein kinases. The remainder of the oncogenes specify proteins that either activate kinases or are phosphorylated by kinases. Although the findings are less direct, aberrant cell signalling through protein kinases has also been associated with cardiovascular disease, diabetes, inflammation, arthritis and other immune disorders, and neurological disorders such as Alzheimer's disease. Over 400 human diseases have been connected to protein kinases. [...]
With the sequencing of the complete human genome, it is now possible to identify all the related genes within distinct families. It is most efficient if a company can develop expertise around a single family of highly similar proteins. Lessons learned from one family member are most rapidly transferable to related proteins. Protein kinases are the largest family of related genes identified so far that encode enzymes with measurable catalytic activities that are suitable to screen for inhibitory drugs. Protein kinases are amongst the most meticulously investigated enzymes by researchers. There is a wealth of data about these enzymes that already serves as a solid foundation from which to build. The primary structures of over 500 human protein kinases are already known and the three-dimensional structures of many different protein kinases have been elucidated. Extensive artificial mutagenesis of several protein kinases has been performed to establish detailed structure-function relationships. After the proteases, protein kinases represent the most attractive candidates for molecular modelling studies to design new drugs. It is estimated that over 25% of the drug discovery efforts in pharmaceutical and biotech companies are focused on protein kinase inhibitors. These drugs have demonstrated applications for treatment of a wide range of diseases including cancer, inflammation, diabetes, congestive heart failure, and neurological damage. Over 60 protein kinase inhibitors are currently in advanced clinical trials, and three are now available in the market place (Herceptin, Gleevec, and Ireesa). The pharmaceutical industry has clearly come to fully recognize the therapeutic potential of protein kinase inhibitors. |
Approximately fifty of the hundred or so known genes that have been directly linked to induction of cancer (i.e. oncogenes) encode protein kinases. The remainder of the oncogenes specify proteins that either activate kinases or are phosphorylated by kinases. Although the findings are less direct, aberrant cell signalling through protein kinases has also been associated with cardiovascular disease, diabetes, inflammation, arthritis and other immune disorders, and neurological disorders such as Alzheimer's disease. Over 400 human diseases have been connected to protein kinases.
[...] With the sequencing of the complete human genome, it is now possible to identify all the related genes within distinct families. It is most efficient if a company can develop expertise around a single family of highly similar proteins. Lessons learned from one family member is most rapidly transferable to related proteins. Protein kinases are the largest family of related genes identified so far that encode enzymes with measurable catalytic activities that are suitable to screen for inhibitory drugs. Protein kinases are amongst the most meticulously investigated enzymes by researchers. There is a wealth of data about these enzymes that already serves as a solid foundation from which to build. The primary structures of over 510 human protein kinases are already known and the three-dimensional structures of many different protein kinases have been elucidated. Extensive artificial mutagenesis of several protein kinases has been performed to establish detailed structure-function relationships. After the proteases, protein kinases represent the most attractive candidates for molecular modelling studies to design new drugs. [...] It is estimated that over 25% of the drug discovery efforts in pharmaceutical and biotech companies are focused on protein kinase inhibitors. These drugs have demonstrated applications for treatment of a wide range of diseases including cancer, inflammation, diabetes, congestive heart failure, and neurological damage. Over 60 protein kinase inhibitors are currently in advanced clinical trials, and three are now available in the market place (Herceptin, Gleevec, and Ireesa). The pharmaceutical industry has clearly come to fully recognize the therapeutic potential of protein kinase inhibitors. |
No source is given. |
|
| [45.] Dsa/Fragment 018 01 - Diskussion Bearbeitet: 2. August 2016, 19:29 WiseWoman Erstellt: 17. May 2015, 18:11 (Hindemith) | Dsa, Fragment, Gesichtet, Kinexus 2006, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 18, Zeilen: 1-13 |
Quelle: Kinexus 2006 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| In the genome of the yeast Saccharomyces cerevisiae, protein kinases represent the largest family of related genes (121 out of 6144 yeast genes encode protein kinases). Many of these kinase genes have mammalian counterparts that will substitute for them in genetically re-engineered yeast. In the fly Drosophila melanogaster, 319 of its 13338 genes encode protein kinases. In the worm C. elegans, 437 of its 18266 genes specify protein kinases. For all of these organisms, this translates to approximately 2% of the total genes corresponding to protein kinases. Recently, the complete genome of the mustard plant Arabidopsis thaliana was reported and it features 1049 putative protein kinases out of 25706 genes. This represented about 4% of that plant's genome. The human genome appears to encode 500 protein kinases in addition to many pseudo-protein kinase genes, and these have been sub classified into over 57 families. There may well be additional protein kinases that remain to be identified. Protein kinases are readily recognized, because they feature characteristic amino acid sequences that distinguish these enzymes from other proteins. | In the genome of the yeast Saccharomyces cerevisiae, protein kinases represent the largest family of related genes (121 out of 6,144 yeast genes encode protein kinases). Many of these kinase genes have mammalian counterparts that will substitute for them in genetically re-engineered yeast. In the fly Drosophila melanogaster, 319 of its 13,338 genes encode protein kinases. In the worm C. elegans, 437 of its 18,266 genes specify protein kinases. For all of these organisms, this translates to approximately 2% of the total genes corresponding to protein kinases. Recently, the complete genome of the mustard plant Arabidopsis thal [sic] was reported, and it features 1049 putative protein kinases out of 25,706 genes. This represented about 4% of that plant's genome. The human genome appears to encode 510 protein kinases in addition to many pseudo-protein kinase genes, and these have been subclassified into over 57 families. There may well be additional protein kinases that remain to be identified. Protein kinases are readily recognized, because they feature characteristic amino acid sequences that distinguish these enzymes from other proteins. |
No source is given. |
|
| [46.] Dsa/Fragment 020 01 - Diskussion Bearbeitet: 2. August 2016, 19:24 WiseWoman Erstellt: 17. May 2015, 17:55 (Hindemith) | Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Wikipedia Serine-threonine-specific protein kinase 2006 |
|
|
| Untersuchte Arbeit: Seite: 20, Zeilen: 1-13 |
Quelle: Wikipedia Serine-threonine-specific protein kinase 2006 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| Structure and autoregulation
The CaM kinases consist of an N-terminal catalytic domain, a regulatory domain and an associative domain. In the absence of Ca2+/calmodulin, the catalytic domain is auto inhibited by the regulatory domain, which contains a pseudo substrate sequence. Several CaM kinases aggregate into a homo-oligomer or hetero-oligomer. Upon activation by Ca2+/calmodulin, the activated CaM kinases autophosphorylate each other, in an intermolecular reaction. This has two effects: • An increase in affinity for the calmodulin complex, prolonging the time the kinase is active. • Continued activation of the phosphorylated kinase complex even after the calmodulin complex has dissociated from the kinase complex, which prolongs the active state even more. |
Structure and autoregulation
The CaM kinases consist of an N-terminal catalytic domain, a regulatory domain, and an association domain. In the absence of Ca2+/calmodulin, the catalytic domain is autoinhibited by the regulatory domain, which contains a pseudosubstrate sequence. Several CaM kinases aggregate into a homooligomer or heterooligomer. Upon activation by Ca2+/calmodulin, the activated CaM kinases autophosphorylate each other in an intermolecular reaction. This has two effects: 1. An increase in affinity for the calmodulin complex, prolonging the time the kinase is active. 2. Continued activation of the phosphorylated kinase complex even after the calmodulin complex has dissociated from the kinase complex, which prolongs the active state even more. |
No source is given. |
|
| [47.] Dsa/Fragment 019 11 - Diskussion Bearbeitet: 2. August 2016, 19:19 WiseWoman Erstellt: 18. May 2015, 18:42 (Hindemith) | Dsa, Fragment, Gesichtet, KidsNet Protein kinase 2007, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 19, Zeilen: 11-23 |
Quelle: KidsNet Protein kinase 2007 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| - Protein kinase C: protein kinase C is actually a family of protein kinases that require Ca2+, diacylglycerol, and a phospholipid such as phosphatidylcholine for activation. Thus, protein kinase C is activated through the same signal transduction pathway as phospholipase C. At least twelve members of the protein kinase C family have been identified in mammals, due to their high sequence homology. The protein kinase C usually means the protein kinase Cα enzyme.
Structure and regulation Protein kinase C enzymes consist of an N-terminal regulatory domain and a C-terminal catalytic domain. The kinases are inactive in the absence of activating agents, due to autoinhibition of the regulatory domain. They can be activated tumor promoters such as tetradecanoyl-phorbol-acetate (TPA) or by the cofactors Ca2+, diacylglycerol and a phospholipid. The common linear structure of protein kinase C enzymes is: N-pseudosubstrate - TPA-binding - (Ca2+-binding) - ATP-binding - substrate-binding- C. |
Protein kinase C
Protein kinase C is actually a family of protein kinases that require Ca2+, diacylglycerol, and a phospholipid such as phosphatidylcholine[?] for activation. Thus, protein kinase C is activated through the same signal transduction pathway as phospholipase C. At least twelve members of the proteine kinase C family have been identified in mammals, due to their high sequence homology[?]. The protein kinase C usually means the protein kinase Cα enzyme. Structure and regulation Protein kinase C enzymes consist of an N-terminal regulatory domain and a C-terminal catalytic domain. The kinases are inactive in the absence of activating agents, due to autoinhibition of the regulatory domain. They can be activated tumor promotors such as tetradecanoyl-phorbol-acetate[?] (TPA) or by the cofactors Ca2+, diacylglycerol, and a phospholipid. The common linear structure of protein kinase C enzymes is: N - pseudosubstrate - TPA-binding - (Ca2+-binding) - ATP-binding - substrate-binding - C |
The source is not given. Note: the text before and after the documented passage can also be found in this source, but other, more likely sources have been used for the documentation of those fragments. |
|
| [48.] Dsa/Fragment 019 35 - Diskussion Bearbeitet: 2. August 2016, 19:12 WiseWoman Erstellt: 17. May 2015, 17:57 (Hindemith) | Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Wikipedia Serine-threonine-specific protein kinase 2006 |
|
|
| Untersuchte Arbeit: Seite: 19, Zeilen: 35-42 |
Quelle: Wikipedia Serine-threonine-specific protein kinase 2006 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| Ca2+/calmodulin-dependent protein kinases:
Also called CaM kinases, these kinases are primarily regulated by the Ca2+/calmodulin complex. These kinases show a memory effect on activation. Two types of CaM kinases are: • Specialized CaM kinases. An example is the myosin light chain kinase (MLCK) that phosphorylates myosin, causing muscles to contract. • Multifunctional CaM kinases. Also collectively called CaM kinase II, which play a role in many processes, such as neurotransmitter secretion, transcription factor regulation, and glycogen metabolism. |
Ca2+/calmodulin-dependent protein kinases
Also called CaM kinases (EC 2.7.11.17), these kinases are primarily regulated by the Ca2+/calmodulin complex. These kinases show a memory effect on activation. Two types of CaM kinases are: • Specialized CaM kinases. An example is the myosin light chain kinase (MLCK) that phosphorylates myosin, causing muscles to contract. • Multifunctional CaM kinases. Also collectively called CaM kinase II, which play a role in many processes, such as neurotransmitter secretion, transcription factor regulation, and glycogen metabolism. |
No source is given. |
|
| [49.] Dsa/Fragment 019 01 - Diskussion Bearbeitet: 2. August 2016, 19:07 WiseWoman Erstellt: 17. May 2015, 17:41 (Hindemith) | Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Wikipedia Protein kinase A 2007 |
|
|
| Untersuchte Arbeit: Seite: 19, Zeilen: 1-10 |
Quelle: Wikipedia Protein kinase A 2007 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| Regulation
Protein kinase A has several functions in the cell, including regulation of glycogen, sugar, and lipid metabolism. It is controlled by cAMP: in the absence of cAMP, the kinase is a tetramer of two regulatory and two catalytic subunits (R2C2), with the regulatory subunits blocking the catalytic center of the catalytic subunits. Binding of cAMP to the regulatory subunit leads to dissociation of active RC dimers. Also, the catalytic subunit itself can be regulated by phosphorylation. Downregulation of protein kinase A occurs by a feedback mechanism: one of the substrates that are activated by the kinase is a phosphodiesterase, which converts cAMP to AMP, thus reducing the amount of cAMP that can activate protein kinase A. |
Regulation
Protein kinase A has several functions in the cell, including regulation of glycogen, sugar, and lipid metabolism. It is controlled by cAMP: in the absence of cAMP, the kinase is a tetramer of two regulatory and two catalytic subunits (R2C2), with the regulatory subunits blocking the catalytic center of the catalytic subunits. Binding of cAMP to the regulatory subunit leads to dissociation of active RC dimers. Also, the catalytic subunit itself can be regulated by phosphorylation. Downregulation of protein kinase A occurs by a feedback mechanism: one of the substrates that is activated by the kinase is a phosphodiesterase, which converts cAMP to AMP, thus reducing the amount of cAMP that can activate protein kinase A. |
No source is given. |
|
| [50.] Dsa/Fragment 019 24 - Diskussion Bearbeitet: 2. August 2016, 19:03 WiseWoman Erstellt: 17. May 2015, 17:37 (Hindemith) | Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Wikipedia Protein kinase C 2007 |
|
|
| Untersuchte Arbeit: Seite: 19, Zeilen: 24-34 |
Quelle: Wikipedia Protein kinase C 2007 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| Upon activation, protein kinase C enzymes are translocated to the plasma membrane by RACK proteins (membrane-bound receptor for activated protein kinase C proteins). The protein kinase C enzymes are known for their long-term activation: they remain activated after the original activation signal or the Ca2+-wave is gone. This is presumably achieved by the production of diacylglycerol from phosphatidylcholine by a phospholipase; fatty acids may also play a role in long-term activation.
Function The consensus sequence of protein kinase C enzymes is similar to that of protein kinase A, since it contains basic amino acids close to the Ser/Thr to be phosphorylated. Their substrates are MARCKS proteins, MAP kinase, transcription factor inhibitor IκB, the vitamin D3 receptor VDR, Raf kinase, calpain, and the EGF receptor. |
Upon activation, protein kinase C enzymes are translocated to the plasma membrane by RACK proteins (membrane-bound receptor for activated protein kinase C proteins). The protein kinase C enzymes are known for their long-term activation: they remain activated after the original activation signal or the Ca2+-wave is gone. This is presumably achieved by the production of diacylglycerol from phosphatidylcholine by a phospholipase; fatty acids may also play a role in long-term activation.
Function The consensus sequence of protein kinase C enzymes is similar to that of protein kinase A, since it contains basic amino acids close to the Ser/Thr to be phosphorylated. Their substrates are MARCKS proteins, MAP kinase, transcription factor inhibitor IκB, the vitamin D3 receptor VDR, Raf kinase, calpain, and the epidermal growth factor receptor. |
No source is given. |
|
| [51.] Dsa/Fragment 021 01 - Diskussion Bearbeitet: 2. August 2016, 13:15 WiseWoman Erstellt: 17. May 2015, 17:22 (Hindemith) | Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Wikipedia Protein kinase 2007 |
|
|
| Untersuchte Arbeit: Seite: 21, Zeilen: 1ff (entire page) |
Quelle: Wikipedia Protein kinase 2007 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| Trans-autophosphorylation (phosphorylation by the other kinase in the dimer) of the kinase.
The autophosphorylation causes the two subdomains of the intrinsic kinase to shift, opening the kinase domain for ATP binding. In the inactive form, the kinase subdomains are aligned so that ATP cannot reach the catalytic center of the kinase. When several amino acids suitable for phosphorylation are present in the kinase domain (e.g., the insulin-like growth factor receptor), the activity of the kinase can increase with the number of phosphorylated amino acids; in this case, the first phosphorylation is said to be a cis-autophosphorylation, switching the kinase from "off" to "standby". Signal transduction The active tyrosine kinase phosphorylates specific target proteins, which are often enzymes themselves. An important target is the ras protein signal-transduction chain. - Receptor-associated tyrosine kinases: Tyrosine kinases recruited to a receptor following hormone binding are receptor-associated tyrosine kinases and are involved in a number of signaling cascades, principally those involved in cytokine signaling (but also others, including growth hormone). One such receptor-associated tyrosine kinase is Janus kinase (JAK), many of whose effects are mediated by STAT proteins. 3) Histidine-specific protein kinases Histidine kinases are structurally distinct from most other protein kinases and are found mostly in prokaryotes as part of two-component signal transduction mechanisms. A phosphate group from ATP is first added to a histidine residue within the kinase, and later transferred to an aspartate residue on a 'receiver domain' on a different protein, or sometimes on the kinase itself. The aspartyl phosphate residue is then active in signaling. Histidine kinases are found widely in prokaryotes, as well as in plants and fungi. The pyruvate dehydrogenase family of kinases in animals is structurally related to histidine kinases, but instead phosphorylate serine residues, and probably do not use a phospho-histidine intermediate. 4) Aspartic acid/glutamic acid-specific protein kinases 5) Mixed kinases Some kinases have mixed kinase activities. For example, MEK (MAPKK), which is involved in the MAP kinase cascade, is a mixed serine/threonine and tyrosine kinase. |
2. Trans-autophosphorylation (phosphorylation by the other kinase in the dimer) of the kinase.
The autophosphorylation causes the two subdomains of the intrinsic kinase to shift, opening the kinase domain for ATP binding. In the inactive form, the kinase subdomains are aligned so that ATP cannot reach the catalytic center of the kinase. When several amino acids suitable for phosphorylation are present in the kinase domain (e.g., the insulin-like growth factor receptor), the activity of the kinase can increase with the number of phosphorylated amino acids; in this case, the first phosphorylation is said to be a cis-autophosphorylation, switching the kinase from "off" to "standby". Signal transduction The active tyrosine kinase phosphorylates specific target proteins, which are often enzymes themselves. An important target is the ras protein signal-transduction chain. Receptor-associated tyrosine kinases Tyrosine kinases recruited to a receptor following hormone binding are receptor-associated tyrosine kinases and are involved in a number of signalling cascades, principally those involved in cytokine signalling (but also others, including growth hormone). One such receptor-associated tyrosine kinase is Janus kinase (JAK), many of whose effects are mediated by STAT proteins. (See JAK-STAT pathway.) Histidine-specific protein kinases Histidine kinases are structurally distinct from most other protein kinases and are found mostly in prokaryotes as part of two-component signal transduction mechanisms. A phosphate group from ATP is first added to a histidine residue within the kinase, and later transferred to an aspartate residue on a 'receiver domain' on a different protein, or sometimes on the kinase itself. The aspartyl phosphate residue is then active in signaling. Histidine kinases are found widely in prokaryotes, as well as in plants and fungi. The pyruvate dehydrogenase family of kinases in animals is structurally related to histidine kinases, but instead phosphorylate serine residues, and probably do not use a phospho-histidine intermediate. Aspartic acid/glutamic acid-specific protein kinases [This section requires expansion.] Mixed kinases Some kinases have mixed kinase activities. For example, MEK (MAPKK), which is involved in the MAP kinase cascade, is a mixed serine/threonine and tyrosine kinase. |
No source is given. The cursive text at the beginning of the page is misunderstood to be a section heading, when it is the second reaction from the previous page. The Wikipedia article has various sizes of bold-face headings |
|
| [52.] Dsa/Fragment 020 14 - Diskussion Bearbeitet: 2. August 2016, 13:08 WiseWoman Erstellt: 17. May 2015, 17:18 (Hindemith) | Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Wikipedia Protein kinase 2007 |
|
|
| Untersuchte Arbeit: Seite: 20, Zeilen: 14-40 |
Quelle: Wikipedia Protein kinase 2007 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| 2) Tyrosine-specific protein kinases
Tyrosine-specific protein kinases phosphorylate tyrosine amino acid residues, and are, like serine/threonine-specific kinases, used in signal transduction. They act primarily as growth factor receptors and in downstream signaling from growth factors; some examples: • Platelet-derived growth factor (PDGF) receptor; • Epidermal growth factor (EGF) receptor; • Insulin receptor and insulin-like growth factor (IGF1) receptor; • Stem cell factor (scf) receptor (also called c-kit). - Receptor tyrosine kinases: These kinases consist of a transmembrane receptor with a tyrosine kinase domain protruding into the cytoplasm. They play an important role in regulating cell division, cellular differentiation, and morphogenesis. More than 50 receptor tyrosine kinases are known in mammals. Structure The extracellular domain serves as the ligand-binding part of the molecule. It can be a separate unit that is attached to the rest of the receptor by a disulfide bond. The same mechanism can be used to bind two receptors together to form a homo- or heterodimer. The transmembrane element is a single α helix. The intracellular or cytoplasmic domain is responsible for the (highly conserved) kinase activity, as well as several regulatory functions. Regulation Ligand binding causes two reactions: • dimerization of two monomeric receptor kinases or • stabilization of a loose dimer. Many ligands of receptor tyrosine kinases are multivalent. Some tyrosine receptor kinases (e.g., the platelet-derived growth factor receptor) can form heterodimers with other similar but not identical kinases of the same subfamily, allowing a highly varied response to the extracellular signal. |
Tyrosine-specific protein kinases
[...] Tyrosine-specific protein kinases (EC 2.7.10.1) phosphorylate tyrosine amino acid residues, and are, like serine/threonine-specific kinases, used in signal transduction. They act primarily as growth factor receptors and in downstream signaling from growth factors; some examples: • Platelet-derived growth factor (PDGF) receptor; • Epidermal growth factor (EGF) receptor; • Insulin receptor and insulin-like growth factor (IGF1) receptor; • Stem cell factor (scf) receptor (also called c-kit, see the article on gastrointestinal stromal tumor). Receptor tyrosine kinases These kinases consist of a transmembrane receptor with a tyrosine kinase domain protruding into the cytoplasm. They play an important role in regulating cell division, cellular differentiation, and morphogenesis. More than 50 receptor tyrosine kinases are known in mammals. Structure The extracellular domain serves as the ligand-binding part of the molecule. It can be a separate unit that is attached to the rest of the receptor by a disulfide bond. The same mechanism can be used to bind two receptors together to form a homo- or heterodimer. The transmembrane element is a single α helix. The intracellular or cytoplasmic domain is responsible for the (highly conserved) kinase activity, as well as several regulatory functions. Regulation Ligand binding causes two reactions: 1. Dimerization of two monomeric receptor kinases or stabilization of a loose dimer. Many ligands of receptor tyrosine kinases are multivalent. Some tyrosine receptor kinases (e.g., the platelet-derived growth factor receptor) can form heterodimers with other similar but not identical kinases of the same subfamily, allowing a highly varied response to the extracellular signal. |
No source is given. The bullet points under Regulation are misleading, as the two reactions are the one on the bottom of page 20 and the one on the top of page 21. |
|
| [53.] Dsa/Fragment 017 01 - Diskussion Bearbeitet: 2. August 2016, 12:33 WiseWoman Erstellt: 17. May 2015, 17:12 (Hindemith) | Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Wikipedia Protein kinase 2007 |
|
|
| Untersuchte Arbeit: Seite: 17, Zeilen: 1-16, 23-24 |
Quelle: Wikipedia Protein kinase 2007 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| 1.2. Protein kinase
A protein kinase is a kinase enzyme that modifies other proteins by chemically adding phosphate groups to them (phosphorylation). This usually results in a functional change of the target protein (substrate) by changing enzyme activity, cellular location, or association with other proteins. Up to 30% of all proteins may be modified by kinase activity, and kinases are known to regulate the majority of cellular pathways, especially those involved in signal transduction, the transmission of signals within the cell. The human genome contains about 500 protein kinase genes; they constitute about 2% of all eukaryotic genes. The chemical activity of a kinase involves removing a phosphate group from ATP and covalently attaching it to one of three amino acids that have a free hydroxyl group. Because protein kinases have profound effects on a cell, their activity is highly regulated. Kinases are turned on or off by phosphorylation (sometimes by the kinase itself - cis-phosphorylation/autophosphorylation), by binding of activator proteins or inhibitor proteins, or small molecules, or by controlling their location in the cell relative to their substrates. Disregulated kinase activity is a frequent cause of disease, particularly cancer, where kinases regulate many aspects that control cell growth, movement and death. [...] Drugs which inhibit specific kinases are being developed to treat several diseases, and some are currently in clinical use. |
Protein kinase
A protein kinase is a kinase enzyme that modifies other proteins by chemically adding phosphate groups to them (phosphorylation). This class of protein is further separated into subsets such as PKC alpha, PKC beta, and PKC gamma, each with specific functions. Phosphorylation usually results in a functional change of the target protein (substrate) by changing enzyme activity, cellular location, or association with other proteins. Up to 30% of all proteins may be modified by kinase activity, and kinases are known to regulate the majority of cellular pathways, especially those involved in signal transduction, the transmission of signals within the cell. The human genome contains about 500 protein kinase genes; they constitute about 2% of all eukaryotic genes. The chemical activity of a kinase involves removing a phosphate group from ATP and covalently attaching it to one of three amino acids that have a free hydroxyl group. Most kinases act on both serine and threonine, others act on tyrosine, and a number (dual specificity kinases) act on all three. Because protein kinases have profound effects on a cell, their activity is highly regulated. Kinases are turned on or off by phosphorylation (sometimes by the kinase itself - cis-phosphorylation/autophosphorylation), by binding of activator proteins or inhibitor proteins, or small molecules, or by controlling their location in the cell relative to their substrates. Disregulated kinase activity is a frequent cause of disease, particularly cancer, where kinases regulate many aspects that control cell growth, movement and death. Drugs which inhibit specific kinases are being developed to treat several diseases, and some are currently in clinical use, including Gleevec (imatinib) and Iressa (gefitinib). |
No source is given. |
|
| [54.] Dsa/Fragment 015 00 - Diskussion Bearbeitet: 2. August 2016, 12:25 WiseWoman Erstellt: 17. May 2015, 17:06 (Hindemith) | Dsa, Fragment, Gesichtet, IvyRose 2001, KomplettPlagiat, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 15, Zeilen: figure |
Quelle: IvyRose 2001 Seite(n): 1 (online source), Zeilen: - |
|---|---|
Figure nr. 2 - Nephron structure. |
Simple Diagram of a Kidney Nephron: |
No source is given. Note, under the figure one finds on the IvyRose website the following statement: "Note: The diagram above is a much simplified representation of a kidney nephron. It includes the same level of detail as the diagram in the Oxford Concise Colour Medical Dictionary and as taught for the ITEC Anatomy & Physiology Course (c.2001). " |
|
| [55.] Dsa/Fragment 016 04 - Diskussion Bearbeitet: 2. August 2016, 09:12 WiseWoman Erstellt: 17. May 2015, 16:45 (Hindemith) | Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Wikipedia Kidney 2007 |
|
|
| Untersuchte Arbeit: Seite: 16, Zeilen: 4-46 |
Quelle: Wikipedia Kidney 2007 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| Excretion of waste products
The kidneys excrete a variety of waste products produced by metabolism, including the nitrogenous wastes: urea (from protein catabolism) and uric acid (from nucleic acid metabolism) and water. Homeostasis The kidney is one of the major organs involved in whole-body homeostasis. Among its homeostatic functions are acid-base balance, regulation of electrolyte concentrations, control of blood volume, and regulation of blood pressure. The kidneys accomplish these homeostatic functions independently and through coordination with other organs, particularly those of the endocrine system. The kidney communicates with these organs through hormones secreted into the bloodstream. Acid-base balance The kidneys regulate the pH, by eliminating H ions concentration called augmentation mineral ion concentration, and water composition of the blood. By exchanging hydronium ions and hydroxyl ions, the blood plasma is maintained by the kidney at a slightly alkaline pH of 7.4. Urine, on the other hand, is acidic at pH 5 or alkaline at pH 8. The pH is maintained through four main protein transporters: NHE3 (a sodium-hydrogen exchanger), V-type H-ATPase (an isoform of the hydrogen ATPase), NBC1 (a sodium-bicarbonate cotransporter) and AE1 (an anion exchanger which exchanges chloride for bicarbonate). Due to the polar alignment of cells in the renal epithelia NHE3 and the H-ATPase are exposed to the lumen (which is essentially outside the body), on the apical side of the cells, and are responsible for excreting hydrogen ions (or protons). Conversely, NBC1 and AE1 are on the basolateral side of the cells, and allow bicarbonate ions to move back into the extracellular fluid and thus are returned to the blood plasma. Blood pressure Sodium ions are controlled in a homeostatic process involving aldosterone which increases sodium ion reabsorption in the distal convoluted tubules.When [sic] blood pressure becomes low, a proteolytic enzyme called Renin is secreted by cells of the juxtaglomerular apparatus (part of the distal convoluted tubule) which are sensitive to pressure. Renin acts on a blood protein, angiotensinogen, converting it to angiotensin I (10 amino acids). Angiotensin I is then converted by the Angiotensin-converting enzyme (ACE) in the lung capillaries to Angiotensin II (8 amino acids), which stimulates the secretion of Aldosterone by the adrenal cortex, which then affects the kidney tubules. Aldosterone stimulates an increase in the reabsorption of sodium ions from the kidney tubules which causes an increase in the volume of water that is reabsorbed from the tubule. This increase in water reabsorption increases the volume of blood which ultimately raises the blood pressure. Plasma volume Any significant rise or drop in plasma osmolality is detected by the hypothalamus, which communicates directly with the posterior pituitary gland. A rise in osmolality causes the gland to secrete antidiuretic hormone, resulting in water reabsorption by the kidney and an increase in urine concentration. The two factors work together to return the plasma osmolality to its normal levels. |
Excretion of waste products
The kidneys excrete a variety of waste products produced by metabolism, including the nitrogenous wastes: urea (from protein catabolism) and uric acid (from nucleic acid metabolism). Homeostasis The kidney is one of the major organs involved in whole-body homeostasis. Among its homeostatic functions are acid-base balance, regulation of electrolyte concentrations, control of blood volume, and regulation of blood pressure. The kidneys accomplish these homeostatic functions independently and through coordination with other organs, particularly those of the endocrine system. The kidney communicates with these organs through hormones secreted into the bloodstream. Acid-base balance The kidneys regulate the pH, by eliminating H ions concentration called augmentation mineral ion concentration, and water composition of the blood. By exchanging hydronium ions and hydroxyl ions, the blood plasma is maintained by the kidney at a slightly alkaline pH of 7.4. Urine, on the other hand, is acidic at pH 5 or alkaline at pH 8. The pH is maintained through four main protein transporters: NHE3 (a sodium-hydrogen exchanger), V-type H-ATPase (an isoform of the hydrogen ATPase), NBC1 (a sodium-bicarbonate cotransporter) and AE1 (an anion exchanger which exchanges chloride for bicarbonate). Due to the polar alignment of cells in the renal epithelia NHE3 and the H-ATPase are exposed to the lumen (which is essentially outside the body), on the apical side of the cells, and are responsible for excreting hydrogen ions (or protons). Conversely, NBC1 and AE1 are on the basolateral side of the cells, and allow bicarbonate ions to move back into the extracellular fluid and thus are returned to the blood plasma.[citation needed] Blood pressure [...] Sodium ions are controlled in a homeostatic process involving aldosterone which increases sodium ion absorption in the distal convoluted tubules. When blood pressure becomes low, a proteolytic enzyme called Renin is secreted by cells of the juxtaglomerular apparatus (part of the distal convoluted tubule) which are sensitive to pressure. Renin acts on a blood protein, angiotensinogen, converting it to angiotensin I (10 amino acids). Angiotensin I is then converted by the Angiotensin-converting enzyme (ACE) in the lung capillaries to Angiotensin II (8 amino acids), which stimulates the secretion of Aldosterone by the adrenal cortex, which then affects the kidney tubules. Aldosterone stimulates an increase in the reabsorption of sodium ions from the kidney tubules which causes an increase in the volume of water that is reabsorbed from the tubule. This increase in water reabsorption increases the volume of blood which ultimately raises the blood pressure. Plasma volume Any significant rise or drop in plasma osmolality is detected by the hypothalamus, which communicates directly with the posterior pituitary gland. A rise in osmolality causes the gland to secrete antidiuretic hormone, resulting in water reabsorption by the kidney and an increase in urine concentration. The two factors work together to return the plasma osmolality to its normal levels. |
The source is not mentioned. |
|
| [56.] Dsa/Fragment 015 01 - Diskussion Bearbeitet: 2. August 2016, 08:19 WiseWoman Erstellt: 17. May 2015, 16:33 (Hindemith) | Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Wikipedia Kidney 2007 |
|
|
| Untersuchte Arbeit: Seite: 15, Zeilen: 1-10 |
Quelle: Wikipedia Kidney 2007 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| Conversely, when the organism must eliminate excess water, such as after excess fluid drinking, the production of ADH is decreased and the collecting tubule becomes less permeable to water, rendering urine dilute and abundant. Failure of the organism to decrease ADH production appropriately, a condition known as syndrome of inappropriate ADH (SIADH), may lead to water retention and dangerous dilution of body fluids, which in turn may cause severe neurological damage. Failure to produce ADH (or inability of the collecting ducts to respond to it) may cause excessive urination, called diabetes insipidus (DI). A second major function of the collecting duct system is the maintenance of acid-base homeostasis. After being processed along the collecting tubules and ducts, the fluid, now called urine, is drained into the bladder via the ureter, to be finally excluded from the organism. | Conversely, when the organism must eliminate excess water, such as after excess fluid drinking, the production of ADH is decreased and the collecting tubule becomes less permeable to water, rendering urine dilute and abundant. Failure of the organism to decrease ADH production appropriately, a condition known as syndrome of inappropriate ADH (SIADH), may lead to water retention and dangerous dilution of body fluids, which in turn may cause severe neurological damage. Failure to produce ADH (or inability of the collecting ducts to respond to it) may cause excessive urination, called diabetes insipidus (DI).
A second major function of the collecting duct system is the maintenance of acid-base homeostasis. After being processed along the collecting tubules and ducts, the fluid, now called urine, is drained into the bladder via the ureter, to be finally excluded from the organism. |
The source is not given. |
|
| [57.] Dsa/Fragment 014 01 - Diskussion Bearbeitet: 2. August 2016, 08:04 WiseWoman Erstellt: 17. May 2015, 16:28 (Hindemith) | Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Wikipedia Kidney 2007 |
|
|
| Untersuchte Arbeit: Seite: 14, Zeilen: 1ff (entire page) |
Quelle: Wikipedia Kidney 2007 Seite(n): 1 (online source), Zeilen: - |
|---|---|
Figure nr. 1 - Kidney structure. Parts of the kidney: 1. Renal pyramid; 2. Efferent vessel; 3. Renal artery; 4. Renal vein; 5. Renal hilum; 6. Renal pelvis; 7. Ureter; 8. Minor calyx; 9. Renal capsule; 10. Inferior renal capsule; 11. Superior renal capsule; 12. Afferent vessel; 13. Nephron; 14. Minor calyx; 15. Major calyx; 16. Renal papilla; 17. Renal column. The basic functional unit of the kidney is the nephron, of which there are more than a million within the cortex and medulla of each normal adult human kidney. Nephrons regulate water and solute within the cortex and medulla of each normal adult human kidney. Nephrons regulate water and soluble matter (especially electrolytes) in the body by first filtering the blood under pressure, and then reabsorbing some necessary fluid and molecules back into the blood while secreting other, unneeded molecules. Reabsorption and secretion are accomplished with both cotransport and countertransport mechanisms established in the nephrons and associated collecting ducts. Collecting duct system The fluid flows from the nephron into the collecting duct system. This segment of the nephron is crucial to the process of water conservation by the organism. In the presence of antidiuretic hormone (ADH; also called vasopressin), these ducts become permeable to water and facilitate its reabsorption, thus concentrating the urine and reducing its volume. |
Parts of the kidney: 1. Renal pyramid 2. Efferent vessel 3. Renal artery 4. Renal vein 5. Renal hilum 6. Renal pelvis 7. Ureter 8. Minor calyx 9. Renal capsule 10. Inferior renal capsule 11. Superior renal capsule 12. Afferent vessel 13. Nephron 14. Minor calyx 15. Major calyx 16. Renal papilla 17. Renal column The basic functional unit of the kidney is the nephron, of which there are more than a million within the cortex and medulla of each normal adult human kidney. Nephrons regulate water and solute within the cortex and medulla of each normal adult human kidney. Nephrons regulate water and soluble matter (especially electrolytes) in the body by first filtering the blood under pressure, and then reabsorbing some necessary fluid and molecules back into the blood while secreting other, unneeded molecules. Reabsorption and secretion are accomplished with both cotransport and countertransport mechanisms established in the nephrons and associated collecting ducts. Collecting duct system [...] The fluid flows from the nephron into the collecting duct system. This segment of the nephron is crucial to the process of water conservation by the organism. In the presence of antidiuretic hormone (ADH; also called vasopressin), these ducts become permeable to water and facilitate its reabsorption, thus concentrating the urine and reducing its volume. |
The source is not mentioned. The illustration is by Piotr Michał Jaworski [2] and is under a CC-BY-SA license, meaning that the illustrator must be credited and any work using it must be under at least a CC-SA license. Thus, in addition to plagiarism, this fragment represents a copyright violation. |
|
| [58.] Dsa/Fragment 013 03 - Diskussion Bearbeitet: 18. March 2016, 14:05 Graf Isolan Erstellt: 17. May 2015, 16:19 (Hindemith) | Dsa, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Wikipedia Kidney 2007 |
|
|
| Untersuchte Arbeit: Seite: 13, Zeilen: 3-40 |
Quelle: Wikipedia Kidney 2007 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| 1. Introduction
1.1. Kidney anatomy The kidneys are organs that filter wastes (such as urea) from the blood and excrete them, along with water, as urine. The medical field that studies the kidneys and diseases of the kidney is called nephrology (nephro- meaning kidney is from the Ancient Greek word nephros; the adjective renal meaning related to the kidney is from Latin rēnēs, meaning kidneys). In humans, the kidneys are located in the posterior part of the abdomen. There is one on each side of the spine; the right kidney sits just below the liver, the left below the diaphragm and adjacent to the spleen. Above each kidney is an adrenal gland (also called the suprarenal gland). The asymmetry within the abdominal cavity caused by the liver results in the right kidney being slightly lower than the left one while the left kidney is located slightly more medial. The kidneys are retroperitoneal, which means they lie behind the peritoneum, the lining of the abdominal cavity. They are approximately at the vertebral level T12 to L3. The upper parts of the kidneys are partially protected by the eleventh and twelfth ribs, and each whole kidney is surrounded by two layers of fat (the perirenal and pararenal fat) which help to cushion it. Congenital absence of one or both kidneys, known as unilateral or bilateral renal agenesis can occur. In a normal human adult, each kidney is about 10 cm long, 5.5 cm in width and about 3 cm thick, weighing 150 grams. Together, kidneys weigh about 0.5% of a person's total body weight. The kidneys are "bean-shaped" organs, and have a concave side facing inwards (medially). On this medial aspect of each kidney is an opening, called the hilum, which admits the renal artery, the renal vein, nerves, and the ureter. The outer portion of the kidney is called the renal cortex, which sits directly beneath the kidney's loose connective tissue/fibrous capsule. Deep to the cortex lies the renal medulla, which is divided into 10-20 renal pyramids in humans. Each pyramid together with the associated overlying cortex forms a renal lobe. The tip of each pyramid (called a papilla) empties into a calyx, and the calices empty into the renal pelvis. The pelvis transmits urine to the urinary bladder via the ureter. Blood supply Each kidney receives its blood supply from the renal artery, two of which branch from the abdominal aorta. Upon entering the hilum of the kidney, the renal artery divides into smaller interlobar arteries situated between the renal papillae. At the outer medulla, the interlobar arteries branch into arcuate arteries, which course along the border between the renal medulla and cortex, giving off still smaller branches, the cortical radial arteries (sometimes called interlobular arteries). Branching off these cortical arteries are the afferent arterioles supplying the glomerular capillaries, which drain into efferent arterioles. Efferent arterioles divide into peritubular capillaries that provide an extensive blood supply to the cortex. Blood from these capillaries collects in renal venules and leaves the kidney via the renal vein. Efferent arterioles of glomeruli closest to the medulla (those that belong to juxtamedullary nephrons) send branches into the medulla, forming the vasa recta. Blood supply is intimately linked to blood pressure. |
In anatomy, urinary system, the kidneys filter wastes (such as urea) from the blood and excrete them, along with water, as urine. The medical field that studies the kidneys and diseases of the kidney is called nephrology (nephro- meaning kidney is from the Ancient Greek word nephros; the adjective renal meaning related to the kidney is from Latin rēnēs, meaning kidneys).
In humans, the kidneys are located in the posterior part of the abdomen. There is one on each side of the spine; the right kidney sits just below the liver, the left below the diaphragm and adjacent to the spleen. Above each kidney is an adrenal gland (also called the suprarenal gland). The asymmetry within the abdominal cavity caused by the liver results in the right kidney being slightly lower than the left one while the left kidney is located slightly more medial. The kidneys are retroperitoneal, which means they lie behind the peritoneum, the lining of the abdominal cavity. They are approximately at the vertebral level T12 to L3. The upper parts of the kidneys are partially protected by the eleventh and twelfth ribs, and each whole kidney is surrounded by two layers of fat (the perirenal and pararenal fat) which help to cushion it. Congenital absence of one or both kidneys, known as unilateral or bilateral renal agenesis can occur. [...] Organization In a normal human adult, each kidney is about 10 cm long, 5.5 cm in width and about 3 cm thick, weighing 150 grams.[1] Together, kidneys weigh about 0.5% of a person's total body weight. The kidneys are "bean-shaped" organs, and have a concave side facing inwards (medially). On this medial aspect of each kidney is an opening, called the hilum, which admits the renal artery, the renal vein, nerves, and the ureter. The outer portion of the kidney is called the renal cortex, which sits directly beneath the kidney's loose connective tissue/fibrous capsule. Deep to the cortex lies the renal medulla, which is divided into 10-20 renal pyramids in humans. Each pyramid together with the associated overlying cortex forms a renal lobe. The tip of each pyramid (called a papilla) empties into a calyx, and the calices empty into the renal pelvis. The pelvis transmits urine to the urinary bladder via the ureter. Blood supply Each kidney receives its blood supply from the renal artery, two of which branch from the abdominal aorta. Upon entering the hilum of the kidney, the renal artery divides into smaller interlobar arteries situated between the renal papillae. At the outer medulla, the interlobar arteries branch into arcuate arteries, which course along the border between the renal medulla and cortex, giving off still smaller branches, the cortical radial arteries (sometimes called interlobular arteries). Branching off these cortical arteries are the afferent arterioles supplying the glomerular capillaries, which drain into efferent arterioles. Efferent arterioles divide into peritubular capillaries that provide an extensive blood supply to the cortex. Blood from these capillaries collects in renal venules and leaves the kidney via the renal vein. Efferent arterioles of glomeruli closest to the medulla (those that belong to juxtamedullary nephrons) send branches into the medulla, forming the vasa recta. Blood supply is intimately linked to blood pressure. 1. Martini F. Fundamentals of Anatomy and Physiology 5th edition. Prentice Hall International Inc. 2001. |
The source is not mentioned. |
|