Permeation of Organometallic Compounds through Phospholipid Membranes
von Raycho Yonchev
Statistik und Sichtungsnachweis dieser Seite findet sich am Artikelende
| [1.] Ry/Fragment 005 01 - Diskussion Zuletzt bearbeitet: 2016-01-23 14:13:08 WiseWoman | Anézo 2003, Fragment, Gesichtet, KomplettPlagiat, Ry, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 5, Zeilen: 1 ff. (entire page) |
Quelle: Anézo 2003 Seite(n): 13, Zeilen: 10ff |
|---|---|
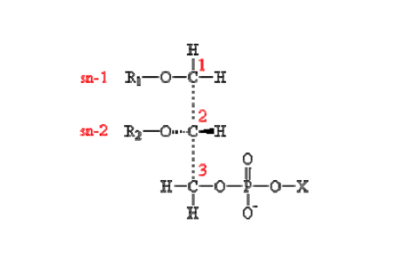
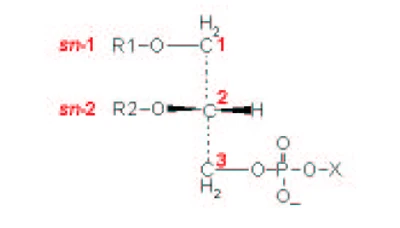
| [A] stereospecific numbering (sn) of the glycerol carbon atoms is commonly used in the nomenclature of glycerophospholipids as indicated on Figure 1.1. In this nomenclature, the two hydrocarbon chains can be differentiated as sn-1 and sn-2 chains, the phosphate group being usually at the sn-3 position of the glycerol. In biological membranes, most of the glycerophospholipids are derivatives of sn-glycero-3-phosphatidic acid, the R-stereoisomer. The various glycerophospholipid types depend on the organic base, amino acid or alcohol (the X-group in Figure 1.2.) to which the phosphate is esterified and on the hydrocarbon chains which can be attached to the glycerol moiety through ester or ether linkages and vary widely in terms of length, branching or degree of unsaturation.
Figure 1.1. General structure of glycerophospholipids with the glycerol backbone drawn in a Fisher [sic] projection. The stereospecific numbering (sn) of the glycerol carbon atoms, with the distinction between the sn-1 and sn-2 chains, is indicated. |
A stereospecific numbering (sn) of the glycerol carbon atoms is commonly used in the nomenclature of glycerophospholipids, as indicated in Figure 1.2. In this nomenclature, the two hydrocarbon chains can be differentiated as sn-1 and sn-2 chains, the phosphate group being usually at the sn-3 position of the glycerol. In biological membranes, most of the glycerophospholipids are derivatives of sn-glycero-3-phosphatidic acid, the R-stereoisomer. The various glycerophospholipid types depend on the organic base, amino acid, or alcohol (the X-group in Figure 1.3) to which the phosphate is esterified and on the hydrocarbon chains which can be attached to the glycerol moiety through ester or ether linkages and vary widely in terms of length, branching, or degree of unsaturation.
Figure 1.2: General structure of glycerophospholipids with the glycerol backbone drawn in a Fischer projection. The stereospecific numbering (sn) of the glycerol carbon atoms, with the distinction between the sn-1 and sn-2 chains, is indicated. |
No source is given. |
|
vorherige Seite | zur Übersichtsseite | folgende Seite
Letzte Bearbeitung dieser Seite: durch Benutzer:WiseWoman, Zeitstempel: 20160123141231
Letzte Bearbeitung dieser Seite: durch Benutzer:WiseWoman, Zeitstempel: 20160123141231