35 gesichtete, geschützte Fragmente: Plagiat
| [1.] Shg/Fragment 013 08 - Diskussion Bearbeitet: 13. December 2014, 20:28 Hindemith Erstellt: 2. November 2014, 20:25 (Hindemith) | Foradori et al 2008, Fragment, Gesichtet, SMWFragment, Schutzlevel sysop, Shg, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 13, Zeilen: 8-13 |
Quelle: Foradori et al 2008 Seite(n): 20, Zeilen: figure 1 |
|---|---|

Figure 1: Androgen actions via intracellular androgen receptor. (1) In the classical pathway. (2) Bound with the SH3 domain. (3) Bound to SHBG. Abbreviations: T, testosterone; DHT, dihydrotestosterone; 5αR, 5alpha reductase enzyme; AR, androgen receptor; PKA, protein kinase A; GP, G-protein; SH2, Src homology domain 2; SH3, Src homology domain 3; PTK, protein tyrosine kinase; MAPK, mitogen-activated protein kinase; SHBGR, steroid hormone-binding globulin receptor; cAMP, cyclic adenosine monophosphate. |
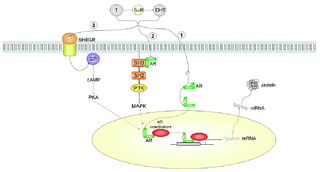
Figure 1. Androgen actions via intracellular androgen receptor mediated pathways. Testosterone (T) can be converted to dihydrotestosterone (DHT) by the 5αR enzyme. 1) In the classical pathway, androgen freely passes through the membrane bi-layer and binds cytoplasmic androgen receptor (AR). Bound AR translocates to the nucleus, binds to a DNA response element on a promoter of an androgen responsive gene and stimulates transcription. 2) Bound AR interacts with the SH3 domain of the tyrosine kinase c-Src to activate the MAPK pathway and influence AR-mediated transcription via phosphorylation of coactivator/ receptor complexes. 3) Androgen bound to steroid hormone binding globulin (SHBG) can activate SHBG receptor (SHBGR) and lead to an increase in PKA activity. PKA may influence AR-mediated transcription via alteration of phosphorylation status of AR and AR coregulators. Abbreviations: T = testosterone, DHT = dihydrotestosterone, 5αR = 5 alpha reductase enzyme, AR = androgen receptor, PKA = protein kinase A, GP = g-protein, SH2 = Src homology domain 2, SH3 = Src homology domain 3, PTK = protein tyrosine kinase, MAPK = mitogen-activated protein kinase, SHBGR = steroid hormone binding globulin receptor, cAMP = cyclic adenosine monophosphate. |
The source is not mentioned here. The figures are not 100% identical, but clearly not independent either. |
|
| [2.] Shg/Fragment 024 08 - Diskussion Bearbeitet: 13. December 2014, 20:28 Hindemith Erstellt: 25. November 2014, 11:51 (Hindemith) | Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Shg, Wang 2010 |
|
|
| Untersuchte Arbeit: Seite: 24, Zeilen: 8-10 |
Quelle: Wang 2010 Seite(n): 1 (online Quelle), Zeilen: - |
|---|---|
| Apoptosis is a form of programmed cell death that plays important roles during animal development, immune response, elimination of damaged cells, and maintenance of tissue homeostasis. | Apoptosis is a form of programmed cell death that plays important roles during animal development, immune response, elimination of damaged cells, and maintenance of tissue homeostasis. |
Die Quelle ist nicht genannt. Im Text der Quelle folgt direkt Shg/Fragment 025 12. |
|
| [3.] Shg/Fragment 025 12 - Diskussion Bearbeitet: 13. December 2014, 20:28 Hindemith Erstellt: 25. November 2014, 11:48 (Hindemith) | Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Shg, Wang 2010 |
|
|
| Untersuchte Arbeit: Seite: 25, Zeilen: 12-18 |
Quelle: Wang 2010 Seite(n): 1 (online Quelle), Zeilen: - |
|---|---|
| Apoptosis is executed by intracellular proteases named caspases that are activated during the onset of apoptosis via extrinsic and intrinsic pathways. The intrinsic pathway is triggered by the release of proteins such as cytochrome c from mitochondria to cytosol and the extrinsic pathway is activated by the binding of death-inducing cytokines such as Tumor Necrosis Factor to its receptor at cell surface. Both pathways are regulated at multiple steps to ensure proper apoptosis. | Apoptosis is executed by intracellular proteases named caspases that are activated during the onset of apoptosis by extrinsic and intrinsic pathways. See more at http://www.ibioseminars.org
The intrinsic pathway is triggered by the release of proteins such as cytochrome c from mitochondria to cytosol and the extrinsic pathway is activated by the binding of death-inducing cytokines such as Tumor Necrosis Factor to its receptor at the cell surface. Both pathways are regulated at multiple steps to ensure proper apoptosis. |
Die Quelle bleibt ungenannt. |
|
| [4.] Shg/Fragment 035 20 - Diskussion Bearbeitet: 13. December 2014, 20:28 Hindemith Erstellt: 2. November 2014, 20:12 (Graf Isolan) | Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Shg, Wikipedia Caco-2 2009 |
|
|
| Untersuchte Arbeit: Seite: 35, Zeilen: 20-22 |
Quelle: Wikipedia Caco-2 2009 Seite(n): 1 (Internetquelle), Zeilen: - |
|---|---|
| The Caco-2 cell line is an immortalized line of heterogeneous human epithelial colorectal adenocarcinoma cells, developed by the Sloan-Kettering Institute for Cancer Research through research conducted by Dr. Jorgen Fogh. | The Caco-2 cell line is an immortalized line of heterogeneous human epithelial colorectal adenocarcinoma cells, developed by the Sloan-Kettering Institute for Cancer Research through research conducted by Dr. Jorgen Fogh. |
Ohne Hinweis auf eine Übernahme. Bemerkenswert ist die Übernahme des akademischen Grads aus der WP in die Dissertation, ein sonst in wissenschaftlichen Texten ganz unübliches Verfahren, das sich auch in der Diss an keiner anderen Stelle findet. |
|
| [5.] Shg/Fragment 043 02 - Diskussion Bearbeitet: 3. November 2014, 19:55 Graf Isolan Erstellt: 2. November 2014, 13:07 (SleepyHollow02) | BauernOpfer, Fragment, Gesichtet, Gu et al 2009, SMWFragment, Schutzlevel sysop, Shg |
|
|
| Untersuchte Arbeit: Seite: 43, Zeilen: 2-3, 11-20 |
Quelle: Gu et al 2009 Seite(n): 3, Zeilen: li. Sp. 11-12, (13-27), 27ff |
|---|---|
| 3.2.4. Immunofluorescence analysis and confocal laser scanning microscopy
For testosterone-HSA-FITC staining, 5-μm-thick frozen tissue sections from the Balb/c or APC mouse tumors were fixed with 4% PFA for 15 min and incubated with 5% BSA/1x PBS/0.3% Triton for 1 hour at room temperature. After two washes with PBS 1.5% FBS specimens were exposed to testosterone-HSA-FITC (10-7 M, Sigma) for 1h at room temperature. Nuclei were stained with DRAQ-5 dye (1:1000, Biostatus, Leicestershire, UK) for 10 min at room temperature. For direct fluorescence microscopy of F-actin, cells were fixed with 3 % paraformaldehyde in PBS for 30 min, permeabilized with 0.5 % Triton X-100 in PBS (10 min) and incubated with rhodamine-phalloidin (Molecular Probes, Eugene, OR, 1:100 dilution) for 40 min in the dark. For indirect immunofluorescence staining, cells were incubated for 2h at room temperature with mouse monoclonal anti-tubulin (Cell signaling, 1: 1000 dilution). Secondary FITC-conjugated rabbit anti-mouse IgG (Invitrogen) was used in a 1: 200 dilution. Nuclei were stained with DRAQ5™ (Biostatus Limited). Slides were mounted using the ProLang® Gold Antifade reagent (Invitrogen). |
Immunofluorescence analysis and confocal laser scanning microscopy
Cells were cultured on glass cover slips with testosterone- HSA-FITC or control HSA-FITC using the concentrations and the incubation periods indicated in the figure legends. For testosterone-HSA-FITC staining, cells or specimens were washed twice with PBS containing 1.5% FBS for 1.5 min and incubated for 1 h with 1% BSA in PBS at room temperature. After two washes with PBS/1.5% FBS cells were exposed to 10-7 M testosterone-HSA-FITC, while control cells were incubated with 4 × 10-7 M HSA-FITC for 1 h at room temperature. Nuclei were stained with DRAQ5™ (Biostatus Limited) or TO-PRO-3 (Invitrogen). After two washes with PBS/1.5% FBS and fixation with 0.5% paraformaldehyde for 30 min cells were washed twice with PBS/1.5% FBS for 3 min and mounted with slow anti-fade. For direct fluorescence microscopy of F-actin, cells were fixed with 3% paraformaldehyde in PBS for 30 min, permeabilized with 0.5% Triton X-100 in PBS (10 min) and incubated with rhodamine-phalloidin (Molecular Probes, Eugene, OR, 1:100 dilution) for 40 min in the dark. For indirect immunofluorescence staining cells were incubated for 2 h at room temperature with mouse monoclonal anti-tubulin (Cell signaling, 1: 1000 dilution). Secondary FITC-conjugated rabbit anti-mouse IgG (Invitrogen) was used at a 1:200 dilution. Nuclei were stained with DRAQ5™ (Biostatus Limited). Slides were mounted using the ProLang® Gold Antifade reagent (Invitrogen). |
Obwohl Shg als Coautor von Gu et al (2009) genannt wird, stammt keine der Formulierungen dieses Artikels von Shg (vgl. die Anmerkungen zu Quelle:Shg/Gu_et_al_2009). Ergo: Insbesondere im zweiten Absatz liegt die Übernahme eines Fremdtextes ohne jede Kennzeichnung vor. Ferner: aus dem "10-7" der Vorlage wird bei Shg "10-7". Gu et al 2009 has been mentioned once on page 41 with respect only to "previous titration experiments". |
|
| [6.] Shg/Fragment 024 10 - Diskussion Bearbeitet: 3. November 2014, 15:34 Hindemith Erstellt: 28. October 2014, 13:36 (SleepyHollow02) | Fragment, Gesichtet, Lawen 2003, SMWFragment, Schutzlevel sysop, Shg, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 24, Zeilen: 10-16 |
Quelle: Lawen 2003 Seite(n): 888, Zeilen: left col., 4 ff., right col., 9 ff. |
|---|---|
| It is associated with a distinct set of biochemical and physical changes involving the cytoplasm, nucleus and plasma membrane. The name was first introduced by John Kerr [Kerr JFR, et al. 1972] in 1972, refers to the morphological feature of formation of ‘‘apoptotic bodies’’ from a cell. Carl Vogt, however, first described the phenomenon more than 100 years earlier in 1842. Now it has become a major research area in the biomedical sciences.
Kerr JFR, Wyllie AH, Currie AR. (1972). Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer;26: 239–571. |
Apoptosis has become a major research area in the biomedical sciences. [...]
The name was first introduced by John Kerr(1) in 1972 and refers to the morphological feature of formation of ‘‘apoptotic bodies’’ from a cell. Carl Vogt, however, first described the phenomenon more than 100 years earlier in 1842. [...] Apoptosis is associated with a distinct set of biochemical and physical changes involving the cytoplasm, nucleus and plasma membrane. 1. Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972;26:239–571. |
Ein Verweis auf die Quelle fehlt hier. |
|
| [7.] Shg/Fragment 059 08 - Diskussion Bearbeitet: 3. November 2014, 15:21 Hindemith Erstellt: 2. November 2014, 15:31 (SleepyHollow02) | Fragment, Gesichtet, Gu et al 2009, SMWFragment, Schutzlevel sysop, Shg, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 59, Zeilen: 8-26 |
Quelle: Gu et al 2009 Seite(n): 6, 7, Zeilen: 6: l.col: 3ff; 7: r.col: 1ff |
|---|---|
| 4.6 mAR activation triggered rapid actin and tubulin reorganization in colon cancer cells
Cytoskeleton reorganization is a prominent early functional response of various cancer cells to steroid hormones targeting membrane binding sites [Koukouritaki et al., 1997, Kampa et al., 2002, Kampa et al., 2006, Papadopoulou et al., 2008a]. Accordingly, to analyze the functional impact of mAR in colon cancer rapid cytoskeleton modifications was investigated in Caco2 cells upon activation of mAR with testosterone-HSA for various time intervals. Cellular actin cytoskeleton dynamics were initially assessed by appropriate quantitative techniques as described in Papakonstanti et al., 2007. As shown in figure 13A, quantitative immunoblot analysis of Triton X-100 insoluble cytoskeletal pellets and corresponding supernatants revealed a significant decrease of the Triton-soluble (monomeric) to total actin ratio in Caco2 cells treated with 10-7 M testosterone-HAS, indicating actin polymerization. This effect was evident 15 min upon testosterone-HSA treatment; and returned to nearly control levels after 1-2 hours (Fig. 13A). The quantitative data were fully supported by confocal laser scanning microscopic analysis, showing redistribution of microfilamentous structures and formation of stress fibers and filopodia in testosterone-HSA treated cells (Fig. 13B). Kampa M, Papakonstanti EA, Hatzoglou A, Stathopoulos EN, Stournaras C, Castanas E. (2002). The human prostate cancer cell line LNCaP bears functional membrane testosterone receptors that increase PSA secretion and modify actin cytoskeleton. Faseb J, 16:1429-1431. Kampa M, Kogia C, Theodoropoulos PA, Anezinis P, Charalampopoulos I, Papakonstanti EA, Stathopoulos EN, Hatzoglou A, Stournaras C, Gravanis A, Castanas E. (2006) Activation of membrane androgen receptors potentiates the antiproliferative effects of paclitaxel on human prostate cancer cells. Mol Cancer Ther, 5:1342-1351. Papadopoulou N, Charalampopoulos I, Alevizopoulos K, Gravanis A, Stournaras C. (2008 a). Rho/ROCK/Actin signaling regualtes membrane androgen receptor induced apoptosis in prostate cancer cells. Exp Cell Res, 314: 3162-3174. |
mAR activation triggered rapid actin and tubulin reorganization in colon cancer cells
Cytoskeleton reorganization is a prominent early functional response of various cancer cells to steroid hormones targeting membrane binding sites [3,8,19,27]. Accordingly to analyze the functional impact of mAR in colon cancer we investigated rapid cytoskeleton modifications in Caco2 cells upon activation of mAR with testosterone- HSA for various time intervals. Cellular actin cytoskeleton dynamics were initially assessed by appropriate quantitative techniques as described in [25]. As shown in fig. 3A, quantitative immunoblot analysis of Triton X-100-insoluble cytoskeletal pellets and correspond- [Seite 7] ing supernatants revealed a significant decrease of the Triton-soluble (monomeric) over total actin ratio in Caco2 cells treated with 10-7 M testosterone-HSA, indicating actin polymerization. This effect was evident 15 min upon testosterone-HSA treatment and returned to nearly control levels after 1-2 h (Fig. 3A). The quantitative data were fully supported by confocal laser scanning microscopic analysis showing redistribution of microfilamentous structures and formation of stress fibers and filopodia in testosterone-HSA treated cells (Fig. 3B). 3. Kampa M, Papakonstanti EA, Hatzoglou A, Stathopoulos EN, Stournaras C, Castanas E: The human prostate cancer cell line LNCaP bears functional membrane testosterone receptors that increase PSA secretion and modify actin cytoskeleton. Faseb J 2002, 16:1429-1431. 8. Papadopoulou N, Charalampopoulos I, Alevizopoulos K, Gravanis A, Stournaras C: Rho/ROCK/Actin signaling regualtes membrane androgen receptor induced apoptosis in prostate cancer cells. Exp Cell Res 2008, 314:3162-3174. 19. Kampa M, Kogia C, Theodoropoulos PA, Anezinis P, Charalampopoulos I, Papakonstanti EA, Stathopoulos EN, Hatzoglou A, Stournaras C, Gravanis A, Castanas E: Activation of membrane androgen receptors potentiates the antiproliferative effects of paclitaxel on human prostate cancer cells. Mol Cancer Ther 2006, 5:1342-1351. 27. Koukouritaki S, Margioris A, Gravanis A, Hartig R, Stournaras C: Dexamethasone induces actin polymerization in human endometrial cells without affecting its synthesis. J Cell Biochem 1997, 65:492-500 |
Die Quelle Koukouritaki fehlt im Literaturverzeichnis. Man beachte die Anmerkung zur Quelle Gu et al. (2009) |
|
| [8.] Shg/Fragment 078 03 - Diskussion Bearbeitet: 3. November 2014, 15:14 Hindemith Erstellt: 1. November 2014, 13:30 (SleepyHollow02) | Fragment, Gesichtet, Gu et al 2009, SMWFragment, Schutzlevel sysop, Shg, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 78, Zeilen: 3-24 |
Quelle: Gu et al 2009 Seite(n): 12, Zeilen: l.col: 32ff |
|---|---|
| 5.2 Membrane androgen receptor activation blocks migration
The connection between actin cytoskeleton components and androgen signaling has attracted specific interest in recent years [Ting HJ, et al. 2008]. Actin dynamics seem to be crucial for apoptotic responses [Gourlay CW, et al.2005, Franklin-Tong VE, et al.2008]. The findings in our present work further underscored the key role of actin cytoskeleton rearrangements in regulating apoptosis. Indeed, it was clearly shown that actin (and tubulin) reorganization represent major early events following mAR activation by testosterone-HSA. Moreover, early blockade of actin rearrangement by depolymerizing drugs e.g. cytochalasin B, virtually abrogated the pro-apoptotic responses (Fig. 15A, B). The involvement of the early actin rearrangement in mediating the late apoptotic responses was addressed in earlier studies in prostate cancer cells. In these studies it was shown that inhibition of either up-stream or down-stream signals regulating early actin polymerization blocked the late activation of NFkB and FasL signaling [Papadopoulou N, et al. 2008A]. Although this pro-apoptotic signaling was not addressed in the present study we hypothesise that the actin reorganization is an early functional step in the pro-apoptotic response. These findings, which are in close agreement with similar results reported recently in prostate cancer cells treated with testosterone albumin conjugates [Papadopoulou N, et al. 2008 and 2008A], further emphasize the functional cross-talk between cytoskeleton rearrangements and regulation of apoptosis [Gourlay CW, et al.2005, Franklin-Tong VE, et al.2008]. Gourlay CW, Ayscough KR. (2005).The actin cytoskeleton: a key regulator of apoptosis and ageing? Nat. Rev. Mol. Cell. Biol, 6(7):6583-589. Franklin-Tong VE, Gourlay CW. (2008). A role for actin in regulating apoptosis/programmed cell death: evidence spanning yeast, plants and animals. Biochem J. 413(3):389-404. Papadopoulou N, Charalampopoulos I, Alevizopoulos K, Gravanis A, Stournaras C. (2008 a). Rho/ROCK/Actin signaling regualtes membrane androgen receptor induced apoptosis in prostate cancer cells. Exp Cell Res, 314: 3162-3174. Papadopoulou N, Charalampopoulos I, Anagnostopoulou V, Konstantinidis G, Föller M, Gravanis A, Alevizopoulos K, Lang F, Stournaras C. (2008 b). Membrane androgen receptor activation triggers down-regulation of PI-3K/Akt/NF-kappaB activity and induces apoptotic responses via Bad, FasL and caspase-3 in DU-145 prostate cancer cells. Mol Canc. 7:88. |
The connection between actin cytoskeleton components and androgen signaling has attracted specific interest in recent years (for a review see [36]). Actin dynamics seem to be crucial for apoptotic responses [28,29]. The findings in our present work further underscored the key role of actin cytoskeleton rearrangements in regulating apoptosis. Indeed, it was clearly shown that actin (and tubulin) reorganization represent major early events following mAR activation by testosterone-HSA. Moreover, early blockade of actin rearrangement by depolymerizing drugs e.g. cytochalasin B, virtually abrogated the pro-apoptotic responses (Fig. 5A, B). The involvement of the early actin rearrangement in mediating the late apoptotic responses was addressed in earlier studies in prostate cancer cells. In these studies it was shown that inhibition of either upstream or down-stream signals regulating early actin polymerization blocked the late activation of NF-κB and FasL signaling [9]. Although the pro-apoptotic signaling was not addressed in the present study we hypothesize that the actin reorganization is an early functional step in the pro-apoptotic response. These findings, which are in close agreement with similar results reported recently in prostate cancer cells treated with testosterone albumin conjugates [8,9], further emphasize the functional crosstalk between cytoskeleton rearrangements and regulation of apoptosis [28,29].
8. Papadopoulou N, Charalampopoulos I, Alevizopoulos K, Gravanis A, Stournaras C: Rho/ROCK/Actin signaling regualtes membrane androgen receptor induced apoptosis in prostate cancer cells. Exp Cell Res 2008, 314:3162-3174. 9. Papadopoulou N, Charalampopoulos I, Anagnostopoulou V, Konstantinidis G, Föller M, Gravanis A, Alevizopoulos K, Lang F, Stournaras C: Membrane androgen receptor activation triggers downregulation of PI-3K/Akt/NF-kappaB activity and induces apoptotic responses via Bad, FasL and caspase-3 in DU-145 prostate cancer cells. Mol Canc 2008, 7:88 28. Gourlay CW, Ayscough KR: The actin cytoskeleton: a key regulator of apoptosis and ageing? Nat Rev Mol Cell Biol 2005, 6(7):6583-589. 29. Franklin-Tong VE, Gourlay CW: A role for actin in regulating apoptosis/programmed cell death: evidence spanning yeast, plants and animals. Biochem J 2008, 413(3):389-404. 36. Ting HJ, Chang C: Actin associated proteins function as androgen receptor coregulators: an implication of androgen receptor's roles in skeletal muscle. J Steroid Biochem Mol Biol 2008, 111(3-5):157-163 |
Die Quelle Ting hat Shg zwar zitiert, aber vergessen im Quellenverzeichnis aufzulisten. Man beachte die Anmerkung zur Quelle Gu et al. (2009) |
|
| [9.] Shg/Fragment 035 04 - Diskussion Bearbeitet: 3. November 2014, 14:57 Hindemith Erstellt: 28. October 2014, 14:48 (SleepyHollow02) | Bailly 2003, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Shg |
|
|
| Untersuchte Arbeit: Seite: 35, Zeilen: 4-18 |
Quelle: Bailly 2003 Seite(n): 163, 165, Zeilen: 163: left col., 27 ff.; 165: left col., 10 ff. |
|---|---|
| Focal complexes are regulated by signaling via Rac1 or cdc42 small GTPases and are marked by the early recruitment of vinculin [J.V. Small et al. 2002 and C.D. Nobes and A. Hall. 1995]. Vinculin is a large protein that contains binding domains for multiple cytoskeletal proteins, including actin, α-actinin, talin, paxillin, VASP, ponsin, vinexin and protein kinase C (PKC) [D.R. Critchley. 2000 and B. Geiger et al. 2001]. Its head and tail regions physically interact in a resting state to mask most binding sites [D.R. Critchley. 2000]. The open, ‘activated’, conformation of vinculin is revealed by exposure to PIP2 and exposes all binding sites. Past studies have revealed that vinculin plays a central role in mechanical coupling of integrins to the cytoskeleton, as well as in the control of cytoskeletal mechanics, cell shape, and protrusion amplitude and cell motility. Vinculin binding to the arp2/3 complex might be but one way that the actin-nucleation machinery can be coupled to new sites of adhesion, and testing this hypothesis now presents cell biologists from different fields with a fascinating new challenge.
Small JV, Stradal T, Vignal E, Rottner K. (2002). The lamellipodium: where motility begins. Trends Cell Biol. 12, pp. 112–120. D.R. Critchley, (2000). Focal adhesions – the cytoskeletal connection. Curr. Opin. Cell Biol. 12, pp. 133–139. C.D. Nobes and A. Hall, (1995). Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, pp. 53–62. Geiger B, Bershadsky A, Pankov R, Yamada KM. (2001).Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2, pp. 793–805. |
Focal complexes are regulated by signaling via Rac1 or cdc42 small GTPases and are marked by the early recruitment of vinculin [3,6]. Vinculin is a large protein that contains binding domains for multiple cytoskeletal proteins, including actin, α-actinin, talin, paxillin, VASP, ponsin, vinexin and protein kinase C (PKC) [7,8]. Its head and tail regions physically interact in a resting state to mask most binding sites [7]. The open, ‘activated’, conformation of vinculin is revealed by exposure to phosphatidylinositol (4,5)-bisphosphate (PIP2) and exposes all binding sites. Past studies have revealed that vinculin plays a central role in mechanical coupling of integrins to the cytoskeleton, as well as in the control of cytoskeletal mechanics, cell shape, protrusion amplitude and cell motility [7].
[Seite 165] Vinculin binding to the arp2/3 complex might be but one way that the actin-nucleation machinery can be coupled to new sites of adhesion, and testing this hypothesis now presents cell biologists from different fields with a fascinating new challenge. 3 Small, J.V. et al. (2002) The lamellipodium: where motility begins. Trends Cell Biol. 12, 112–120 6 Nobes, C.D. and Hall, A. (1995) Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53–62 7 Critchley, D.R. (2000) Focal adhesions – the cytoskeletal connection. Curr. Opin. Cell Biol. 12, 133–139 8 Geiger, B. et al. (2001) Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2, 793–805 |
Ein Verweis auf die Quelle fehlt. |
|
| [10.] Shg/Fragment 012 21 - Diskussion Bearbeitet: 3. November 2014, 14:52 Hindemith Erstellt: 25. October 2014, 05:41 (SleepyHollow02) | Foradori et al 2008, Fragment, Gesichtet, SMWFragment, Schutzlevel sysop, Shg, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 12, Zeilen: 21-25 |
Quelle: Foradori et al 2008 Seite(n): 1, Zeilen: 21ff |
|---|---|
| The classic genomic model for steroid hormone action presumes that steroid hormones can freely cross the Plasma Membrane, enter the cytoplasm, and bind to activate specific iAR. The bound steroid receptors act as transcription factors and bind as homodimers or heterodimers to specific DNA response elements in target gene promoters, causing protein synthesis. [Guido M, Uta C.P. 2008]
Guido M, Uta C.P. (2008). Raptid action of androgens. Neuroendocrinology 29: 182-198. |
This classic genomic model for steroid hormone action presumes that steroid hormones can freely cross the plasma membrane, enter the cytoplasm, and bind to and activate specific intracellular steroid receptor proteins. The bound steroid receptors act as transcription factors and bind as homodimers or heterodimers to specific DNA response elements in target gene promoters, causing activation or repression of transcription and subsequently protein synthesis (Figure 1) [2; 3; 4; 5; 6].
2. Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–344. [PubMed] 3. Roy AK, Lavrovsky Y, Song CS, Chen S, Jung MH, Velu NK, Bi BY, Chatterjee B. Regulation of androgen action. Vitam Horm. 1999;55:309–352. [PubMed] 4. Heinlein CA, Chang C. Androgen receptor (AR) coregulators: an overview. Endocr Rev. 2002;23:175–200. [PubMed] 5. Zhou ZX, Wong CI, Sar M, Wilson EM. The androgen receptor: an overview. Recent Prog Horm Res. 1994;49:249–274. [PubMed] 6. Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev. 1995;16:271–321. [PubMed] |
Ein Verweis auf die Quelle fehlt. Die angegebene Quelle enthält die Passage nicht. Man beachte dass mit "Guido M, Uta C.P. (2008). Raptid action of androgens. Neuroendocrinology 29: 182-198." wohl "Guido Michels, Uta C. Hoppe (2008). Rapid actions of androgens. Frontiers in Neuroendocrinology 29 (2008) 182–198" gemeint ist. |
|
| [11.] Shg/Fragment 025 01 - Diskussion Bearbeitet: 2. November 2014, 20:33 Hindemith Erstellt: 28. October 2014, 14:38 (SleepyHollow02) | Fragment, Gesichtet, Gewies 2003, SMWFragment, Schutzlevel sysop, Shg, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 25, Zeilen: 1-9 |
Quelle: Gewies 2003 Seite(n): 4, Zeilen: 18-27 |
|---|---|
| [Those morphological changes are consequences of] characteristic molecular and biochemical events which occur in an apoptotic cell. Most of them are activated notably by proteolytic enzymes. It finally mediates the cleavage of DNA into oligonucleosomal fragments, in at the same time as the cleavage of a multitude of specific protein substrates which usually determine the integrity and shape of the cytoplasm or organelles [Saraste, A; Pulkki, K 2000]. Furthermore, apoptosis is in contrast to the necrotic mode of cell-death. During necrosis, the cellular contents are released uncontrolled into the cell's environment, which results in damage of surrounding cells and a strong inflammatory response in the corresponding tissue.
Saraste, A and Pulkki, K (2000). "Morphologic and biochemical hallmarks of apoptosis." Cardiovasc Res 45(3): 528-37. |
Those morphological changes are a consequence of characteristic molecular and biochemical events occurring in an apoptotic cell, most notably the activation of proteolytic enzymes which eventually mediate the cleavage of DNA into oligonucleosomal fragments as well as the cleavage of a multitude of specific protein substrates which usually determine the integrity and shape of the cytoplasm or organelles [Saraste, 2000]. Apoptosis is in contrast to the necrotic mode of cell-death in which case the cells suffer a major insult, resulting in a loss of membrane integrity, swelling and disrupture of the cells. During necrosis, the cellular contents are released uncontrolled into the cell's environment which results in damage of surrounding cells and a strong inflammatory response in the corresponding tissue [Leist, 2001a].
Saraste, A and Pulkki, K (2000). "Morphologic and biochemical hallmarks of apoptosis." Cardiovasc Res 45(3): 528-37. Leist, M and Jaattela, M (2001). "Four deaths and a funeral: from caspases to alternative mechanisms." Nat. Rev. Mol. Cell Biol. 2(8): 589-98. |
Ohne Hinweis auf eine Übernahme. |
|
| [12.] Shg/Fragment 016 05 - Diskussion Bearbeitet: 2. November 2014, 20:10 Hindemith Erstellt: 2. November 2014, 19:03 (Graf Isolan) | Fragment, Gesichtet, SABiosciences Androgen Signaling 2009, SMWFragment, Schutzlevel sysop, Shg, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 16, Zeilen: 5-30 |
Quelle: SABiosciences Androgen Signaling 2009 Seite(n): 1 (Internetquelle), Zeilen: - |
|---|---|
| Nongenomic steroid activity involves the rapid induction of conventional second messenger signal transduction cascades. Nongenomic action of androgens can occur through multiple receptors. Androgens activate cAMP and PKA through membrane androgen receptor (mAR). Androgens also induce an elevation in intracellular Ca2+ through mAR to a GPCR (G-Protein Coupled Receptor) by activating an influx through nonvoltage-gated Ca2+ channels. The increasing of intracellular calcium activates signal transduction cascades, which is included PKA (Protein Kinase-A), PKC (Protein Kinase-C), and MAPKs (Mitogen-Activated Protein Kinase). They can modulate the activity of the ARs and other transcription factors. AR can also interact with the intracellular tyrosine kinase c-Src, triggering c-Src activation. One of the targets of c-Src is the adapter protein SHC (SH2 Containing Protein). It is an upstream regulator of the MAPK pathway. The activation of AR are influenced by direct phosphorylation by MAPK [Heinlein CA, Chang C. 2002]. In another side, AR phosphorylation by ERK2 is associated with enhanced AR transcriptional activity and an increased ability to recruit the coactivator ARA70.[Heinlein CA, Chang C. 2002] The SRC family of transcriptional coactivators includes SRC1, SRC3, and TIF2 (Transcription Intermediary Factor-2). They are targets of MAPK phosphorylation and result in an increased ability of these coactivators to recruit additional coactivator complexes to the DNA-bound receptor. The nongenomic, rapid stimulation of second messenger cascades by androgens may ultimately exert biological effects through modulation of the transcriptional activity of AR or other transcription factors. Those modulations may happen by direct phosphorylation of transcriptional activators or their coregulators [Michels G, Hoppe UC. 2008]. In the absence of AR’s cognate ligand the AR can also be activated. Androgen can initiate by various growth factors.
Culig Z, Klocker H, Bartsch G, Steiner H, Hobisch A. (2003). Androgen Receptors in Prostate Cancer. J Urol. Oct.170(4):1363-1369. Heinlein CA, Chang C. (2002). The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol 16:2181–2187. Michels G, Hoppe UC. (2008). Rapid actions of androgens. Front Neuroendocrinol. May;29(2):182-98. |
Nongenomic steroid activity typically involves the rapid induction of conventional second messenger signal transduction cascades. Nongenomic action of androgens can occur through multiple receptors. Androgens can activate cAMP and PKA through the SHBG (Sex Hormone Binding Globulin)/SHBGR complex (Ref.1). Androgens also stimulate an elevation in intracellular Ca2+ through a GPCR (G-Protein Coupled Receptor) by activating an influx through nonvoltage-gated Ca2+ channels. The elevation of intracellular calcium activates signal transduction cascades, including PKA (Protein Kinase-A), PKC (Protein Kinase-C), and MAPKs (Mitogen-Activated Protein Kinase), that can modulate the activity of the ARs and other transcription factors. AR also interacts with the intracellular tyrosine kinase c-Src, triggering c-Src activation. One of the targets of c-Src is the adapter protein SHC (SH2 Containing Protein), an upstream regulator of the MAPK pathway. The activity of AR and AR coactivators are influenced by direct phosphorylation by MAPK (Ref.3). AR phosphorylation by ERK2 is associated with enhanced AR transcriptional activity and an increased ability to recruit the coactivator ARA70. The SRC family of transcriptional coactivators: SRC1, SRC3, and TIF2 (Transcription Intermediary Factor-2) are targets of MAPK phosphorylation that results in an increased ability of these coactivators to recruit additional coactivator complexes to the DNA-bound receptor. The nongenomic, rapid stimulation of second messenger cascades by androgens may ultimately exert biological effects through modulation of the transcriptional activity of AR or other transcription factors. Such modulation may occur through direct phosphorylation of transcriptional activators or their coregulators (Ref.1). The AR can also be activated in the absence of its cognate ligand, androgen by signaling pathways initiated by various growth factors.
[...] References: 1. Heinlein CA, Chang C 3. Culig Z, Klocker H, Bartsch G, Steiner H, Hobisch A |
Ohne Hinweis auf eine Übernahme. "Culig Z, Klocker H, Bartsch G, Steiner H, Hobisch A. (2003)" wird nicht ein einziges Mal in der Dissertation von Shg erwähnt und taucht erst im Literaturverzeichnis auf. |
|
| [13.] Shg/Fragment 023 04 - Diskussion Bearbeitet: 2. November 2014, 20:06 Hindemith Erstellt: 23. October 2014, 00:23 (Graf Isolan) | DErrico und Moschetta 2008, Fragment, Gesichtet, SMWFragment, Schutzlevel sysop, Shg, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 23, Zeilen: 4-21 |
Quelle: DErrico und Moschetta 2008 Seite(n): 1534, Zeilen: li.Sp. 43-47, re.Sp. 11-18.21-35 |
|---|---|
| 1.2.4. Colon cancer and steroid receptors
Although colon cancer is not a hormone-dependent tumor, the existence of sex differences in colon cancer incidence was proposed several years ago. The activity of steroid receptor plays a pivotal role in a well-controlled cascade of signals, which maintains the mucosal architecture by the shedding of senescent and apoptotic cells at the surface of the epithelium. The identification of a functional interaction between the Wnt/APC pathway and steroid represents a major goal for several laboratories [Mulholland, et al 2005]. β-Catenin activates a growing number of steroid receptors, resulting in alterations of cell proliferation and tumorigenesis. On the other hand, Wnt signaling appears to be compromised by the action of some steroid receptors. It is also clear that steroid receptors are regionally compartmentalized along the cryptvillus axis, determining the switching on and off of transcription of particular genes with a strong influence on cell fate. The mechanism for the influence of steroid receptors on cell proliferation, differentiation and apoptosis in the gut is complex and still under investigation. Also, the observed phenotypes after steroid receptor activation or inhibition are sometimes contradictory. Steroid receptor effects depend on the amount of agonists, the cell type and the mutational. [Mulholland, et al. 2005] Mulholland, D. J., Dedhar, S., Coetzee, G. A. and Nelson, C. C. (2005). Interaction of nuclear receptors with the Wnt/ beta-catenin/Tcf signaling axis: Wnt you like to know? Endocr. Rev. 26, 898–915. |
The existence of sex differences in colon cancer incidence was proposed several years ago, due to the observation that this neoplasia occurs more often in men than in women in nearly all countries [256]. [...]
[...] [...] A well-controlled cascade of signals maintains the mucosal architecture by the shedding of senescent and apoptotic cells at the surface of the epithelium. The activity of some NRs seems to play a pivotal role in this process. The identification of a functional interaction between the Wnt/APC pathway and NRs represents a major goal for several laboratories [260]. [...] What we know today is that β-catenin activates a growing number ofNRs, resulting in alterations of cell proliferation and tumorigenesis. On the other hand, Wnt signaling appears to be compromised by the action of some NRs. It is also clear that NRs are regionally compartmentalized along the cryptvillus axis, determining the switching on and off of transcription of particular genes with a strong influence on cell fate. The mechanism for the influence of NRs on cell proliferation, differentiation and apoptosis in the gut is complex and still under investigation. Also, the observed phenotypes after NR activation or inhibition are sometimes contradictory. NR effects depend on the amount of agonists, on the cell type and on the mutational events that predispose cells to cancer development. 256 Haenszel, W. and Correa, P. (1971) Cancer of the colon and rectum and adenomatous polyps. A review of epidemiologic findings. Cancer 28, 14–24. 260 Mulholland, D. J., Dedhar, S., Coetzee, G. A. and Nelson, C. C. (2005) Interaction of nuclear receptors with the Wnt/ beta-catenin/Tcf signaling axis: Wnt you like to know? Endocr. Rev. 26, 898–915. |
Ohne Hinweis auf eine Übernahme. Der letzte Satz bricht unvermittelt ab und wird so teilweise unverständlich. In der ungenannt bleibenden Quelle liegt der Satz in unverstümmelter Form vor. "NR" in der Quelle steht für "Nuclear receptor". Die Referenz zu Mulholland et al 2005 wurde aus dem Literaturverzeichnis der Quelle kopiert - das überzählige Leerzeichen zwischen "/" und "beta-catenin/" (was sich dahinter nicht findet) entspringt einem Zeilenumbruch im Original. Mulholland, et al. (2005) enthält den Text nicht. |
|
| [14.] Shg/Fragment 032 01 - Diskussion Bearbeitet: 2. November 2014, 15:37 Graf Isolan Erstellt: 26. October 2014, 14:21 (Graf Isolan) | Fragment, Gesichtet, KomplettPlagiat, Manning und Cantley 2007, SMWFragment, Schutzlevel sysop, Shg |
|
|
| Untersuchte Arbeit: Seite: 32, Zeilen: 1-3 |
Quelle: Manning und Cantley 2007 Seite(n): 1269, Zeilen: re.Sp. 17-20 |
|---|---|
| These studies demonstrate both the importance of crosstalk between the PI3K-Akt pathway and other pathways and the emerging recognition that the three isoforms of Akt can have distinct cellular functions. | These studies demonstrate both the importance of crosstalk between the PI3K-Akt pathway and other pathways and the emerging recognition that the three isoforms of Akt can have distinct cellular functions. |
Ohne Hinweis auf eine Übernahme; schließt die auf der vorangegangenen Seite begonnene Übernahme ab. |
|
| [15.] Shg/Fragment 020 01 - Diskussion Bearbeitet: 2. November 2014, 15:35 Graf Isolan Erstellt: 30. October 2014, 13:33 (Graf Isolan) | BauernOpfer, Fragment, Gesichtet, Gu et al 2009, SMWFragment, Schutzlevel sysop, Shg |
|
|
| Untersuchte Arbeit: Seite: 20, Zeilen: 1-8, 10-21 |
Quelle: Gu et al 2009 Seite(n): 2, Zeilen: li.Sp. 10-19, 24-32, 41-53 - re.Sp. 1- |
|---|---|
| [Taken together, these studies clearly established that functional mARs trigger strong anti-tumorigenic effects in prostate and breast cancer cells, implying a] potential role of mAR as a novel target for the development of selective cancer treatments [Papadopoulou et al., 2009]. It was shown that mAR activation resulted in actin reorganization regulated by distinct mechanisms involving small GTPases’ specific signaling cascades. [Papadopoulou N, et al 2008b] Furthermore, it was shown that mAR activation induced potent apoptotic regression of prostate cancer cells in vitro [Papadopoulou N, et al 2008a] and in mouse xenografts in vivo and suppressed cell growth and motility. [Hatzoglou A, et al 2005, Kampa et al 2006] [...] However, it remained elusive whether mARs were also expressed in other tumors and whether their activation could result in the induction of anti-tumorigenic effects similar to the ones described in prostate and breast cancer cells.
In my diploma thesis, by using either colon cancer tissues isolated from mice xenograft tumors or two established colon cancer cell lines (Caco2 and HCT116 cells), the expression and function of functional role of mAR has been analyzed. As a result, testosterone binding sites were expressed in the membrane of colon cancer cells and qualify as bona fide membrane androgen receptors as assessed by radioligand binding studies, Scatchard analysis and displacement assays. The activation of those receptors with non permeable testosterone derivatives induced pro-apoptotic responses. [Gu S, et al. 2009]. Gu S, Papadopoulou N, Gehring EM, Nasir O, Dimas K, Bhavsar SK, Foller M, Alevizopoulos K, Lang F, Stournaras C. (2009) Functional membrane androgen receptors in colon tumors trigger pro-apoptotic responses in vitro and reduce drastically tumor incidence in vivo. Mol Cancer. 8:114. Hatzoglou A, Kampa M, Kogia C, Charalampopoulos I, Theodoropoulos PA, Anezinis P, Dambaki C, Papakonstanti EA, Stathopoulos EN, Stournaras C, et al. (2005). Membrane androgen receptor activation induces apoptotic regression of human prostate cancer cells in vitro and in vivo. J Clin Endocrinol Metab, 90: 893-903. Kampa M, Kogia C, Theodoropoulos PA, Anezinis P, Charalampopoulos I, Papakonstanti EA, Stathopoulos EN, Hatzoglou A, Stournaras C, Gravanis A, Castanas E. (2006) Activation of membrane androgen receptors potentiates the antiproliferative effects of paclitaxel on human prostate cancer cells. Mol Cancer Ther, 5:1342-1351. Papadopoulou N, Charalampopoulos I, Alevizopoulos K, Gravanis A, Stournaras C. (2008 a). Rho/ROCK/Actin signaling regualtes membrane androgen receptor induced apoptosis in prostate cancer cells. Exp Cell Res, 314: 3162-3174. Papadopoulou N, Charalampopoulos I, Anagnostopoulou V, Konstantinidis G, Föller M, Gravanis A, Alevizopoulos K, Lang F, Stournaras C. (2008 b). Membrane androgen receptor activation triggers down-regulation of PI-3K/Akt/NF-kappaB activity and induces apoptotic responses via Bad, FasL and caspase-3 in DU-145 prostate cancer cells. Mol Canc. 7:88. Papadopoulou N, Papakonstanti EA, Kallergi G, Alevizopoulos K, Stournaras C. (2009). Membrane androgen receptor activation in prostate and breast tumor cells: Molecular signaling and clinical impact. IUBMB Life, 61(1): 56-61. |
The mAR-dependent signaling was recently characterized in detail in prostate and breast cancer cell lines (reviewed in [14-17]). Using non-permeable androgen derivatives that do not bind to iAR, it was shown that mAR activation resulted in actin reorganization regulated by mechanisms involving small GTPases [8,18]. Furthermore, it was shown that mAR activation induced profound apoptotic regression of prostate cancer cells in vitro and in mouse xenografts in vivo [7,19] and suppressed cell growth and motility [6,19]. [...]
Taken together, these studies clearly established that functional mARs trigger strong anti-tumorigenic effects, implying a potential role of mAR as a novel target for the development of selective cancer treatments (reviewed in [17]). However, it remained elusive whether mARs are also expressed in other tumors and whether their activation could result in the induction of anti-tumorigenic effects similar to the ones described in prostate and breast cancer cells. [...] Since the membrane androgen receptor, in contrast to the classical intracellular androgen receptor, induces tumor regression in target tissues (reviewed in [17]), we sought to determine the expression and functional status of mAR in colon cancer. To this end, we used colon cancer tissues isolated from mice xenograft tumors and from two established colon cancer cell lines (Caco2 and HCT116 cells). As a result, testosterone binding sites were expressed in the membrane of colon cancer cells and qualify as bona fide membrane androgen receptors as assessed by radioligand binding studies, Scatchard analysis and displacement assays. The activation of those receptors with nonpermeable testosterone derivatives triggered rapid and profound actin and tubulin cytoskeleton reorganization and induced pro-apoptotic responses. 6. Kallergi G, Agelaki S, Markomanolaki H, Georgoulias V, Stournaras C: Activation of FAK/PI3K/Rac1 signaling controls actin reorganization and inhibits cell motility in human cancer cells. Cell Physiol Biochem 2007, 20:977-986. 7. Hatzoglou A, Kampa M, Kogia C, Charalampopoulos I, Theodoropoulos PA, Anezinis P, Dambaki C, Papakonstanti EA, Stathopoulos EN, Stournaras C, Gravanis A, Castanas E: Membrane androgen receptor activation induces apoptotic regression of human prostate cancer cells in vitro and in vivo. J Clin Endocrinol Metab 2005, 90:893-903. 8. Papadopoulou N, Charalampopoulos I, Alevizopoulos K, Gravanis A, Stournaras C: Rho/ROCK/Actin signaling regualtes membrane androgen receptor induced apoptosis in prostate cancer cells. Exp Cell Res 2008, 314:3162-3174. 9. Papadopoulou N, Charalampopoulos I, Anagnostopoulou V, Konstantinidis G, Föller M, Gravanis A, Alevizopoulos K, Lang F, Stournaras C: Membrane androgen receptor activation triggers down-regulation of PI-3K/Akt/NF-kappaB activity and induces apoptotic responses via Bad, FasL and caspase-3 in DU-145 prostate cancer cells. Mol Canc 2008, 7:88. 14. Kampa M, Pelekanou V, Castanas E: Membrane-initiated steroid action in breast and prostate cancer. Steroids 2008, 73(9-10):953-960. 15. Michels G, Hoppe UC: Rapid actions of androgens Front Neuroendocrinol 2008, 29(2):182-198. 16. Foradori CD, Weiser MJ, Handa RJ: Non-genomic actions of androgens. Front Neuroendocrinol 2008, 29(2):169-181. 17. Papadopoulou N, Papakonstanti EA, Kallergi G, Alevizopoulos K, Stournaras C: Membrane androgen receptor activation in prostate and breast tumor cells: Molecular signaling and clinical impact. IUBMB Life 2009, 61(1):56-61. 18. Papakonstanti EA, Kampa M, Castanas E, Stournaras C: A rapid, nongenomic, signaling pathway regulates the actin reorganization induced by activation of membrane testosterone receptors. Mol Endocrinol 2003, 17:870-881. 19. Kampa M, Kogia C, Theodoropoulos PA, Anezinis P, Charalampopoulos I, Papakonstanti EA, Stathopoulos EN, Hatzoglou A, Stournaras C, Gravanis A, Castanas E: Activation of membrane androgen receptors potentiates the antiproliferative effects of paclitaxel on human prostate cancer cells. Mol Cancer Ther 2006, 5:1342-1351. |
Obwohl Shg als Coautor von Gu et al (2009) genannt wird, stammt keine der Formulierungen dieses Artikels von Shg (vgl. die Anmerkungen zu Quelle:Shg/Gu_et_al_2009). Ergo: Übernahme von Formulierungen eines Fremdtextes (inkl. aller dort zu findenden Literaturreferenzen) ohne jede Kennzeichnung. Durch den Vergleich ergibt sich auch (implizit), dass Shg die an dieser Stelle in Gu et al (2009) beschriebenen Untersuchungen als Inhalt ihrer "Diploma thesis" ansieht. |
|
| [16.] Shg/Fragment 041 01 - Diskussion Bearbeitet: 2. November 2014, 13:08 Singulus Erstellt: 1. November 2014, 22:50 (Graf Isolan) | BauernOpfer, Fragment, Gesichtet, Gu et al 2009, SMWFragment, Schutzlevel sysop, Shg |
|
|
| Untersuchte Arbeit: Seite: 41, Zeilen: 1-5, 10-26 |
Quelle: Gu et al 2009 Seite(n): 2, 4, Zeilen: 2: re.Sp. 9-24, 28-36; 4: re.Sp. 7-12 |
|---|---|
| 3.2 Methods
3.2.1. Cell culture The Caco2 human colon cancer cell lines and IEC06 non transformed intestinal cells were obtained from the American Type Culture Collection (Manassas, VA) and were studied between passages 60 and 70. CACO2 at 20,000/ml were cultured in DEME medium supplemented with 20% fetal bovine serum in culture flasks in a CO2 incubator at 37°C. Based on previous titration experiments [Gu et al., 2009] we have used throughout this study a 10-7 M testosterone-HAS concentration for mAR stimulation. 3.2.2. Preparation of steroid solution Before each experiment testosterone-3- (O-carboxymethyl) oxime-Human Serum Albumin, referred to as testosterone-HSA (or Testo-HSA), DHT and estradiol, were dissolved in serum-free culture medium at a final concentration of 10-5 M. This stock solution was incubated for 30 min at room temperature with 0.3% charcoal and 0.03% dextran, centrifuged at 3000 x g and passed through a 0.45 μm filter to remove any potential contamination with free steroid. Testosterone-HSA, estradiol and DHT solutions were used at a final concentration of 10-7 M throughout all studies. If not otherwise stated all treatments and incubations with steroids including apoptosis assays were performed in serum-containing medium. Testosterone-HSA-FITC or control HSA-FITC constructs were generated by conjugating Testosterone-HSA or HSA with FITC using standard techniques. 3.2.3. In vivo animal experiment Colon carcinoma was generated as described previously (Wang et al., 2004). In a first series of experiments, 7-week old Balb/c mice (both male and female) were divided into two groups, A (n=5) and B (n=7). Both groups [underwent carcinogenic treatment.] |
[Seite 2]
Materials and methods Cell cultures The Caco2 and HCT116 human colon cancer cell lines and the non transformed intestinal IEC06 cells were obtained from the American Type Culture Collection (Manassas, VA) and were studied between passages 55 and 70. Preparation of steroid solution Before each experiment testosterone-3-(O-carboxymethyl) oxime human serum albumin, (testosterone-HSA or Testo-HSA; Sigma) was dissolved in serum-free culture medium at a final concentration of 10-5 M. This stock solution was incubated for 30 min at room temperature with 0.3% charcoal and 0.03% dextran, centrifuged at 3,000 x g and passed through a 0.45 μm filter to remove any potential contamination with free steroid. This is highly important for the interpretation of the results to disconnect any possible intracellular testosterone- and/or iAR-interference with the effects mainly induced by the mAR activation. Testosterone HSA, estradiol and dihydrotestosterone (DHT) (Sigma) solutions were used at a final concentration of 10-7 M throughout the study unless otherwise mentioned. All treatments and incubations with steroids including apoptosis assays were performed in serum-containing medium. Testosterone-HSA-FITC or control HSA-FITC constructs were generated by conjugating Testosterone-HSA or HSA with FITC (Sigma) using standard techniques. [Seite 4] Induction of colon carcinoma Colon carcinoma was generated as described previously [26]. In a first series of experiments, 7-week old Balb/c mice (both male and female) were divided into two groups, A (n = 5) and B (n = 7). Both groups underwent carcinogenic treatment. 26. Wang JG, Wang DF, Lv BJ, Si JM: A novel mouse model for colitis-associated colon carcinogenesis induced by 1,2-dimethylhydrazine and dextran sulfate sodium. World J Gastroenterol 2004, 10:2958-2962. |
Obwohl Shg als Coautor von Gu et al (2009) genannt wird, stammt keine der Formulierungen dieses Artikels von Shg (vgl. die Anmerkungen zu Quelle:Shg/Gu_et_al_2009). Ergo: Übernahme eines Fremdtextes ohne jede Kennzeichnung. Ferner: aus dem "10-7" der Vorlage wird bei Shg in der Einleitung "10-7". Eine Referenz für "(Wang et al., 2004)" fehlt im Literaturverzeichnis von Shg. |
|
| [17.] Shg/Fragment 022 13 - Diskussion Bearbeitet: 2. November 2014, 09:01 Hindemith Erstellt: 23. October 2014, 09:38 (SleepyHollow02) | BauernOpfer, Fragment, Gesichtet, Li Lai 2009, SMWFragment, Schutzlevel sysop, Shg |
|
|
| Untersuchte Arbeit: Seite: 22, Zeilen: 13-32 |
Quelle: Li Lai 2009 Seite(n): 221, 222, Zeilen: 221: right col., 17 ff.; 222: left col.: 1ff |
|---|---|
| 1.2.3. Genes
There is much progress which has been made in understanding the molecular mechanism of colorectal cancer. A progression from normal mucosa to adenoma to carcinoma was supported by the demonstration of accumulating mutations in genes as K-ras, adenomatous polyposis coli (APC), tumor protein P53 (TP53), and deleted in DCC, all of which are thought to be of significance, but are not able to successfully account for all colorectal cancers. There is heterogeneity in the pathogenetic pathway leading to CRCs, and there are two major tumorigenic pathways. The first is driven by chromosomal instability (CIN), the progress of which involves both oncogenes and tumor-suppressor genes including chromosomes 5q, 17p, and 18q [Fearon, E.R., Vogelstein, B. 1990; Gervaz, P., et al. 2001]. Chromosome 5q genes are responsible for APC, 17p for TP53, and 18q for DCC or Mothers against decapentaplegic homolog 4 (SMAD4). K-ras is the most common oncogene following this pattern. The tumor-suppressor genes APC, TP53, and DCC/SMAD4 play important roles in this sequential adenoma to carcinoma. Another genetic pathway may well be depicted as a consequence of the alteration in mismatch repair (MMR) genes. [Gervaz, P., et al. 2001] When the alteration happens in germinal cells, the hereditary cancer known as hereditary nonpolyposis colorectal cancer (HNPCC) occurs. When somatic cells are affected, microsatellite instability (MSI) would be [unavoidable. MSI is responsible for a subset of sporadic colorectal tumors. [Feng-ying LI, Mao-de LAI 2008]] Fearon, E.R., Vogelstein, B. (1990). A genetic model for colorectal tumorigenesis. Cell, 61(5):759-76. Gervaz, P., Bouzourene, H., Cerottini, J.P., Chaubert, P., Benhattar, J., Secic, M., Wexner, S., Givel, J.C., Belin, B. (2001). Dukes B colorectal cancer: distinct genetic categories and clinical outcome based on proximal or distal tumor location. Dis. Colon Rectum. 44(3):364-372. Feng-ying LI, Mao-de LAI (2008) Colorectal cancer, one entity or three. J Zhejiang Univ Sci. B 10(3):219-229. |
GENETIC PATHWAY
Much progress has been made in understanding the molecular mechanism of CRC since 1990, when a genetic model for CRC tumorigenesis was proposed (Fearon and Vogelstein, 1990). A progression from normal mucosa to adenoma to carcinoma was supported by the demonstration of accumulating mutations in genes of K-ras, adenomatous polyposis coli (APC), tumor protein P53 (TP53), and deleted in colorectal carcinoma (DCC), all of which are thought to be of significance, but are not able successfully to account for all CRCs. There is heterogeneity in the pathogenetic pathway leading to CRCs, and there are two major tumorigenic pathways. The first is driven by chromosomal instability (CIN), namely the model mentioned above, the progress of which involves both oncogenes and tumor-suppressor genes including chromosomes 5q, 17p, and 18q (Delattre et al., 1989; Fearon and Vogelstein, 1990; Gervaz et al., 2001). Chromosome 5q genes are responsible for APC, 17p for TP53, and 18q for DCC or Mothers against decapentaplegic homolog 4 (SMAD4), respectively. K-ras is the most common oncogene following this pattern. As far as tumor-suppressor genes are concerned, genes of APC, TP53, DCC/SMAD4 play important roles in this sequential adenoma to carcinoma pattern. An alternative genetic pathway related to genetic instability may well be depicted as a consequence of [Page 222] the alteration in mismatch repair (MMR) genes (Gervaz et al., 2001; Miyakura et al., 2001; Thibodeau et al., 1993). When the alteration happens in germinal cells, the hereditary cancer known as hereditary nonpolyposis colorectal cancer (HNPCC) occurs. When somatic cells are affected, microsatellite instability (MSI) would be unavoidable, which is responsible for a subset of sporadic colorectal tumors. Delattre, O., Olschwang, S., Law, D.J., Melot, T., Remvikos, Y., Salmon, R.J., Sastre, X., Validire, P., Feinberg, A.P., Thomas, G., 1989. Multiple genetic alterations in distal and proximal colorectal cancer. Lancet, 334(8659):353-356. [doi:10.1016/S0140-6736(89)90537-0] Fearon, E.R., Vogelstein, B., 1990. A genetic model for colorectal tumorigenesis. Cell, 61(5):759-767. [doi:10.1016/0092-8674(90)90186-I] Gervaz, P., Bouzourene, H., Cerottini, J.P., Chaubert, P., Benhattar, J., Secic, M., Wexner, S., Givel, J.C., Belin, B., 2001. Dukes B colorectal cancer: distinct genetic categories and clinical outcome based on proximal or distal tumor location. Dis. Colon Rectum, 44(3):364-372. [doi:10.1007/BF02234734] Miyakura, Y., Sugano, K., Konishi, F., Ichikawa, A., Maekawa, M., Shitoh, K., Igarashi, S., Kotake, K., Koyama, Y., Nagai, H., 2001. Extensive methylation of hMLH1 promoter region predominates in proximal colon cancer with microsatellite instability. Gastroenterology, 121(6): 1300-1309. [doi:10.1053/gast.2001.29616] Thibodeau, S.N., Bren, G., Schaid, D., 1993. Microsatellite instability in cancer of the proximal colon. Science, 260(5109):816-819. [doi:10.1126/science.8484122] |
The source is mentioned in the end, but it does not become clear that the entire section including to references to the literature is taken from it. |
|
| [18.] Shg/Fragment 021 18 - Diskussion Bearbeitet: 2. November 2014, 08:57 Hindemith Erstellt: 23. October 2014, 09:31 (SleepyHollow02) | Fragment, Gesichtet, Li Lai 2009, SMWFragment, Schutzlevel sysop, Shg, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 21, Zeilen: 18-27 |
Quelle: Li Lai 2009 Seite(n): 219, Zeilen: right col., 3 ff. |
|---|---|
| According to the Surveillance, Epidemiology and End Results (SEER) Program database analysis, 5-year survival rates have risen from 56.5% for patients diagnosed in the early 1980s to as much as 63.2% for those diagnosed in the early 1990s and most recently to 64.9%, a trend due mostly to earlier diagnosis and treatment [Ries LAG, et al. 2008]. One reason for the improving trend is that the prognosis for patients with CRC is highly dependent on stage: 5-year survival rates are over 90% for Dukes A, but only 5% for Dukes D. Unfortunately, only 10% of CRCs are diagnosed early, most patients presenting themselves with the advanced disease [Rockville, MD. 1998].
Ries LAG, Melbert D, Krapcho M, et al. (2008). SEER Cancer Statistics Review. National Cancer Institute: 1975-2005. Rockville, MD. (1998). Agency for Health Care Policy and Research. AHCPR Publication No. 98-003. |
According to the Surveillance, Epidemiology and End Results (SEER) Program database analysis, 5-year survival rates have risen from 56.5% for patients diagnosed in the early 1980s to as much as 63.2% for those diagnosed in the early 1990s and most recently to 64.9%, a trend due mostly to earlier diagnosis and treatment (Ries et al., 2008). One reason for the improving trend is that the prognosis for patients with CRC is highly dependent on stage: 5-year survival rates are over 90% for Dukes A, but only 5% for Dukes D. Unfortunately, only 10% of CRCs are diagnosed early, most patients presenting themselves with advanced disease (AHCPR, 1998)
Ries, L.A.G., Melbert, D., Krapcho, M., Stinchcomb, D.G., Howlader, N., Horner, M.J., Mariotto, A., Miller, B.A., Feuer, E.J., Altekruse, S.F., Lewis, D.R., et al. (Eds.), 2008. SEER Cancer Statistics Review, 1975-2005 [WWW]. National Cancer Institute. Available from: http://seer.cancer.gov/csr/1975_2005/results_merged/topic_survival.pdf [Accessed 06/08/2008]. AHCPR (Agency for Health Care Policy and Research), 1998. Colorectal Cancer Screening. Technical Review 1. AHCPR Publication No. 98-0033. Agency for Health Care Policy and Research, Rockville, MD. |
The source is not mentioned here. |
|
| [19.] Shg/Fragment 019 01 - Diskussion Bearbeitet: 2. November 2014, 08:51 Hindemith Erstellt: 22. October 2014, 16:16 (SleepyHollow02) | BauernOpfer, Fragment, Gesichtet, Papadopoulou et al 2009, SMWFragment, Schutzlevel sysop, Shg |
|
|
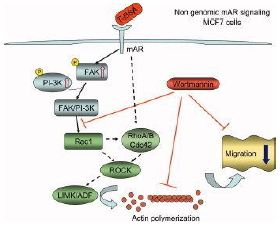
| Untersuchte Arbeit: Seite: 19, Zeilen: 1-19 |
Quelle: Papadopoulou et al 2009 Seite(n): 58, Zeilen: left col., 3 ff. |
|---|---|
| [Solid arrows] indicate events that have been experimentally proven. Dashed arrows indicate unidentified possible links. See text for details. [Papadopoulou N, et al 2009]
In breast cancer, it has been reported that mAR is expressed in T47D and MCF7 human breast epithelial cancer cells. In T47D cells, specific and saturable androgen receptors are present in the membrane and their activation via TBSA conjugates resultes [sic] in cell death by apoptosis. [Kampa M, et al 2005] Moreover, pharmacological inhibitors of MEK and p38 kinase were able to block T-BSA induced apoptosis showing a functional implication of these pathways in mAR-dependent apoptosis in T47D cells. However, in MCF7 cells, activation of these receptors by T-BSA conjugates triggered a non-genomic signaling pathway involving FAK and PI-3K phosphorylation and downstream activation of the small GTPase Rac1, ultimately resulting in actin redistribution. Cell migration experiments provided insights in the functional role of mAR stimulation in MCF7 cells. But the activations of mAR did not induce any apoptotic response in this kind of cells. [Kallergi G, et a.l 2007] Figure 4: mAR signaling in breast epithelial cancer cells Non-genomic mAR signaling operating in MCF7 breast epithelial cancer cells regulating actin redistribution and cell motility. Solid arrows indicate events that have been experimentally proven. Dashed arrows indicate unidentified possible links. [Papadopoulou N, et al 2009] Papadopoulou N, Papakonstanti EA, Kallergi G, Alevizopoulos K, Stournaras C. (2009). Membrane androgen receptor activation in prostate and breast tumor cells: Molecular signaling and clinical impact. IUBMB Life, 61(1): 56-61. Kampa M, Nifli AP, Charalampopoulos I, Alexaki VI, Theodoropoulos PA, Stathopoulos EN, Gravanis A, Castanas E. (2005). Opposing effects of estradiol- and testosterone-membrane binding sites on T47D breast cancer cell apoptosis. Exp Cell Res, 307:41-51. Kallergi G, Agelaki S, Markomanolaki H, Georgoulias V, Stournaras C. (2007). Activation of FAK/PI3K/Rac1 signaling controls actin reorganization and inhibits cell motility in human cancer cells. Cell Physiol Biochem, 20:977-986. |
Figure 2. Early and late mAR signaling operating in iAR deficient DU145 human prostate cancer cells regulating actin redistribution, downstream pro-apoptotic signaling, and migration. Solid arrows indicate events that have been experimentally proven. Dashed arrows indicate unidentified possible links. See text for details.
Figure 3. Non-genomic mAR signaling operating in MCF7 breast epithelial cancer cells regulating actin redistribution and cell motility. Solid arrows indicate events that have been experimentally proven. Dashed arrows indicate unidentified possible links. See text for details. Besides prostate cancer cells, mAR expression has recently been reported in T47D and MCF7 human breast epithelial cancer cells. In T47D cells, specific and saturable androgen receptors are present in the membrane and their activation via TBSA conjugates resulted in cell death by apoptosis (15). [...] Moreover, pharmacological inhibitors of MEK and p38 kinase were able to block T-BSA induced apoptosis showing a functional implication of these pathways in mAR-dependent apoptosis in T47D cells. In another recent study, we have reported the expression of mAR in MCF7 cells (20). Activation of these receptors by T-BSA conjugates triggered a non-genomic signaling pathway involving FAK and PI-3K phosphorylation and downstream activation of the small GTPase Rac1, ultimately resulting in actin redistribution. [...] Cell migration experiments provided insights in the functional role of mAR stimulation in MCF7 cells. [...]
15. Kampa, M., Nifli, A. P., Charalampopoulos, I., Alexaki, V. I., Theodoropoulos, P.A., Stathopoulos, E.N., Gravanis, A., and Castanas, E. (2005) Opposing effects of estradiol- and testosterone-membrane binding sites on T47D breast cancer cell apoptosis. Exp. Cell. Res. 307, 41–51. 20. Kallergi, G., Agelaki, S., Markomanolaki, H., Georgoulias, V., and Stournaras, C. (2007) Activation of FAK/PI3K/Rac1 signaling controls actin reorganization and inhibits cell motility in human cancer cells. Cell. Physiol. Biochem. 20, 977–986. |
Although the source is given for the figure nothing has been marked as a citation. |
|
| [20.] Shg/Fragment 031 10 - Diskussion Bearbeitet: 2. November 2014, 01:28 Hindemith Erstellt: 26. October 2014, 01:02 (Graf Isolan) | Fragment, Gesichtet, KomplettPlagiat, Manning und Cantley 2007, SMWFragment, Schutzlevel sysop, Shg |
|
|
| Untersuchte Arbeit: Seite: 31, Zeilen: 10-32 |
Quelle: Manning und Cantley 2007 Seite(n): 1269, Zeilen: li.Sp.43 ff. - re.Sp. 1-17 |
|---|---|
| A role for Akt in the control of cell migration, invasion of the extracellular matrix, and ultimately metastasis has been difficult to ascertain. Strikingly, activation of Akt1 has been found to decrease mammary epithelial cell migration, and Akt1 prevents an epithelial-to-mesenchymal transition that resembles events required for metastasis [Irie et al., 2005] and [Yoeli-Lerner et al., 2005]. Two independent mechanisms for this surprising Akt function have been explored. The first found that the inhibitory effect of Akt1 on the in vitro migration and invasion properties of breast cancer cell lines involved a pathway leading to degradation of the nuclear factor of activated T cells (NFAT) transcription factors [Yoeli-Lerner et al., 2005]. However, the molecular mechanism of Akt1-mediated degradation of NFAT is currently unknown. A second group found that siRNA knockdown of Akt1, but not Akt2, led to an increase in the migration of mammary epithelial cells [Irie et al., 2005]. Loss of Akt1, specifically, led to an increase in the activation of Erk1 and Erk2, which was found to be required for the enhanced migration. Again, the mechanism by which Akt1, but not Akt2, inhibits Erk signaling in this system remains unknown. Interestingly, mouse tumor models have also suggested that Akt1 inhibits metastases [Hutchinson et al., 2004], whereas Akt2 promotes metastases [Arboleda et al., 2003]. However, these differential effects of Akt1 and Akt2 on epithelial cell migration may not translate to other cell types. In fact, studies on cell migration using mouse embryonic fibroblasts deficient of specific Akt isoforms have suggested opposite effects on fibroblast migration, with Akt1 promoting migration and with Akt2 inhibiting it [ [Zhou et al., 2006].]
G.L. Zhou, D.F. Tucker, S.S. Bae, K. Bhatheja, M.J. Birnbaum and J. Field. (2006). Opposing roles for Akt1 and Akt2 in Rac/Pak signaling and cell migration, J. Biol. Chem. 281 ,pp. 36443–36453. Hutchinson, J. Jin, R.D. Cardiff, J.R. Woodgett and W.J. Muller. (2004). Activation of Akt-1 (PKB-alpha) can accelerate ErbB-2-mediated mammary tumorigenesis but suppresses tumor invasion, Cancer Res. 64 (2004), pp. 3171–3178. M.J. Arboleda, J.F. Lyons, F.F. Kabbinavar, M.R. Bray, B.E. Snow, R. Ayala, M. Danino, B.Y. Karlan and D.J. Slamon,. (2003). Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells, Cancer Res. 63 pp. 196–206. Yoeli-Lerner, G.K. Yiu, I. Rabinovitz, P. Erhardt, S. Jauliac and A. Toker. (2005). Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT, Mol. Cell 20, pp. 539–550. |
Cell Migration and Invasion
Until recently, a role for Akt in the control of cell migration, invasion of the extracellular matrix, and ultimately metastasis has been difficult to ascertain. Strikingly, activation of Akt1 has been found to decrease mammary epithelial cell migration, and Akt1 prevents an epithelial-to-mesenchymal transition that resembles events required for metastasis (Irie et al., 2005; Yoeli-Lerner et al., 2005). Two independent mechanisms for this surprising Akt function have been explored. The first found that the inhibitory effect of Akt1 on the in vitro migration and invasion properties of breast cancer cell lines involved a pathway leading to degradation of the nuclear factor of activated T cells (NFAT) transcription factors (Yoeli-Lerner et al., 2005). However, the molecular mechanism of Akt1-mediated degradation of NFAT is currently unknown. A second group found that siRNA knockdown of Akt1, but not Akt2, led to an increase in the migration of mammary epithelial cells (Irie et al., 2005). Loss of Akt1, specifically, led to an increase in the activation of Erk1 and Erk2, which was found to be required for the enhanced migration. Again, the mechanism by which Akt1, but not Akt2, inhibits Erk signaling in this system remains unknown. Interestingly, mouse tumor models have also suggested that Akt1 inhibits metastases (Hutchinson et al., 2004), whereas Akt2 promotes metastases (Arboleda et al., 2003). However, these differential effects of Akt1 and Akt2 on epithelial cell migration may not translate to other cell types. In fact, studies on cell migration using mouse embryonic fibroblasts deficient of specific Akt isoforms have suggested opposite effects on fibroblast migration, with Akt1 promoting migration and with Akt2 inhibiting it (Zhou et al., 2006). Arboleda, M.J., Lyons, J.F., Kabbinavar, F.F., Bray, M.R., Snow, B.E., Ayala, R., Danino, M., Karlan, B.Y., and Slamon, D.J. (2003). Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res. 63, 196–206. Hutchinson, J.N., Jin, J., Cardiff, R.D., Woodgett, J.R., and Muller, W.J. (2004). Activation of Akt-1 (PKB-alpha) can accelerate ErbB-2- mediated mammary tumorigenesis but suppresses tumor invasion. Cancer Res. 64, 3171–3178. Irie, H.Y., Pearline, R.V., Grueneberg, D., Hsia, M., Ravichandran, P., Kothari, N., Natesan, S., and Brugge, J.S. (2005). Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J. Cell Biol. 171, 1023–1034. Yoeli-Lerner, M., Yiu, G.K., Rabinovitz, I., Erhardt, P., Jauliac, S., and Toker, A. (2005). Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol. Cell 20, 539–550. Zhou, G.L., Tucker, D.F., Bae, S.S., Bhatheja, K., Birnbaum, M.J., and Field, J. (2006). Opposing roles for Akt1 and Akt2 in Rac/Pak signaling and cell migration. J. Biol. Chem. 281, 36443–36453. |
Ohne Hinweis auf eine Übernahme. Eine Referenz für [Irie et al., 2005] fehlt im Literaturverzeichnis von Shg. |
|
| [21.] Shg/Fragment 034 13 - Diskussion Bearbeitet: 2. November 2014, 01:28 Hindemith Erstellt: 27. October 2014, 19:07 (Graf Isolan) | Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Saarikangas et al 2010, Schutzlevel sysop, Shg |
|
|
| Untersuchte Arbeit: Seite: 34, Zeilen: 13-33 |
Quelle: Saarikangas et al 2010 Seite(n): 263, 270, Zeilen: 263: li.Sp. 51-52 - re.Sp. 1-17; 270: re.Sp. 26-28 - 271: li.Sp. 1 ff. |
|---|---|
| The organization and dynamics of the actin cytoskeleton are regulated by membrane phosphoinositides at several levels. First, many actin-binding proteins directly interact with phosphoinositides, which regulate the activity and/or subcellular localization of these proteins. Among different PIs, PIP2 is the best-characterized regulator of the actin cytoskeleton. PIP2 interacts directly with several actin-binding proteins and regulates their activities [Hilpela P, et al.2004, Sechi AS, Wehland J. 2000, Sheetz MP, et al. 2006, Yamaguchi H, et al. 2009]. Typically, PIP2 inhibits those actin-binding proteins that promote actin filament disassembly and activates proteins that induce actin filament assembly. Second, phosphoinositides control the subcellular localization of larger scaffolding proteins that are involved in the interplay between the actin cytoskeleton and plasma membrane or intracellular membrane organelles. Finally, proteins controlling the activity of Rho family small GTPases are in many cases regulated by plasma membrane phosphoinositides. The RhoA GTPase has a pronounced role in the formation and regulation of focal adhesion complexes and contractile actomyosin bundles such as stress fibers [Pelham RJ, et al. 1994]. RhoA induces actin polymerization at focal adhesions by activating the Dia1 formin and inhibits actin filament disassembly by initiating a signaling cascade that leads to phosphorylation and subsequent inactivation of the ADF/cofilin family of actin filament severing/depolymerizing proteins through the action of LIM kinases [Hotulainen P, Lappalainen P. 2006, Mahaffy [RE, Pollard TD. 2008, Watanabe N, et al. 1999, Vardouli et al 2005].]
Hilpela P, Vartiainen MK, Lappalainen P. (2004). Regulation of the actin cytoskeleton by PI(4,5)P2 and PI(3,4,5)P3. Curr Top Microbiol Immunol 282: 117–163. Hotulainen P, Lappalainen P. (2006). Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol 173: 383–394. Mahaffy RE, Pollard TD. (2008). Influence of phalloidin on the formation of actin filament branches by Arp2/3 complex. Biochemistry 47: 6460–6467. Pelham RJ, Chang F. (2002). Actin dynamics in the contractile ring during cytokinesis in fission yeast. Nature 419: 82–86. Sechi AS, Wehland J. (2000).The actin cytoskeleton and plasma membrane connection: PtdIns(4,5)P2 influences cytoskeletal protein activity at the plasma membrane. J Cell Sci 113: 3685–3695. Sheetz MP, Sable JE, Dobereiner HG. (2006). Continuous membrane-cytoskeleton adhesion requires continuous accommodation to lipid and cytoskeleton dynamics. Annu Rev Biophys Biomol Struct 35: 417–434. Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S. (1999). Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol 1: 136–143. Yamaguchi H, Shiraishi M, Fukami K, Tanabe A, Ikeda-Matsuo Y, Naito Y, Sasaki Y. (2009). MARCKS regulates lamellipodia formation induced by IGF-I via association with PIP2 and beta-actin at membrane microdomains. J Cell Physiol 220: 748–755. |
[Seite 263]
II. REGULATION OF ACTIN DYNAMICS BY PHOSPHOINOSITIDES The organization and dynamics of the actin cytoskeleton are regulated by membrane phosphoinositides at several levels. First, many actin-binding proteins directly interact with phosphoinositides, which regulate the activity and/or subcellular localization of these proteins. Second, phosphoinositides control the subcellular localization of larger scaffolding proteins that are involved in the interplay between the actin cytoskeleton and plasma membrane or intracellular membrane organelles. Finally, proteins controlling the activity of Rho family small GTPases are in many cases regulated by plasma membrane phosphoinositides. Among different PIs, PI(4,5)P2 is the best-characterized regulator of the actin cytoskeleton. PI(4,5)P2 interacts directly with several actin-binding proteins and regulates their activities (23, 141, 330, 332, 397). Typically, PI(4,5)P2 inhibits those actin-binding proteins that promote actin filament disassembly and activates proteins that induce actin filament assembly. [Seite 270] A. Rho Family GTPases The RhoA GTPase has a pronounced role in the formation and regulation of focal adhesion complexes and contractile actomyosin bundles such as stress fibers (288, 308). RhoA induces actin polymerization at focal adhesions by activating the Dia1 formin and inhibits actin filament disassembly by initiating a signaling cascade that leads to phosphorylation and subsequent inactivation of the ADF/cofilin family of actin filament severing/depolymerizing proteins through the action of LIM kinases (146, 230, 383). 23. Bittar EE. (Editor). Advances in Molecular and Cell Biology. New York: Elsevier, 2006. 141. Hilpela P, Vartiainen MK, Lappalainen P. Regulation of the actin cytoskeleton by PI(4,5)P2 and PI(3,4,5)P3. Curr Top Microbiol Immunol 282: 117–163, 2004. 146. Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol 173: 383–394, 2006. 230. Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 285: 895–898, 1999. 231. Mahaffy RE, Pollard TD. Influence of phalloidin on the formation of actin filament branches by Arp2/3 complex. Biochemistry 47: 6460–6467, 2008. 288. Pelham RJ, Chang F. Actin dynamics in the contractile ring during cytokinesis in fission yeast. Nature 419: 82–86, 2002. 308. Ridley AJ, Hall A. Signal transduction pathways regulating Rhomediated stress fibre formation: requirement for a tyrosine kinase. EMBO J 13: 2600–2610, 1994. 330. Sechi AS, Wehland J. The actin cytoskeleton and plasma membrane connection: PtdIns(4,5)P2 influences cytoskeletal protein activity at the plasma membrane. J Cell Sci 113: 3685–3695, 2000. 332. Sheetz MP, Sable JE, Dobereiner HG. Continuous membrane-cytoskeleton adhesion requires continuous accommodation to lipid and cytoskeleton dynamics. Annu Rev Biophys Biomol Struct 35: 417–434, 2006. 383. Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol 1: 136–143, 1999. 397. Yamaguchi H, Shiraishi M, Fukami K, Tanabe A, Ikeda-Matsuo Y, Naito Y, Sasaki Y. MARCKS regulates lamellipodia formation induced by IGF-I via association with PIP2 and beta-actin at membrane microdomains. J Cell Physiol 220: 748–755, 2009. |
Ohne Hinweis auf eine Übernahme. Im Literaturverzeichnis von Shg findet sich keine Referenz für "Vardouli et al 2005". Beim Versuch, die Referenz [230] zu kopieren, hat sich Shg vertan und stattdessen die unter [231] stehende Referenz übernommen. |
|
| [22.] Shg/Fragment 035 01 - Diskussion Bearbeitet: 2. November 2014, 01:26 Hindemith Erstellt: 27. October 2014, 21:07 (Graf Isolan) | Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Saarikangas et al 2010, Schutzlevel sysop, Shg |
|
|
| Untersuchte Arbeit: Seite: 35, Zeilen: 1-3 |
Quelle: Saarikangas et al 2010 Seite(n): 271, Zeilen: li.Sp. 1-9 |
|---|---|
| [RhoA induces actin polymerization at focal adhesions by activating the Dia1 formin and inhibits actin filament disassembly by initiating a signaling cascade that leads to phosphorylation and subsequent inactivation of the ADF/cofilin family of actin filament severing/depolymerizing proteins through the action of LIM kinases [Hotulainen P, Lappalainen P. 2006, Mahaffy] RE, Pollard TD. 2008, Watanabe N, et al. 1999, Vardouli et al 2005]. Furthermore, RhoA promotes contractility by activating the myosin light-chain kinase through ROCK kinase [Totsukawa G, et al. 2000].
Hotulainen P, Lappalainen P. (2006). Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol 173: 383–394. Mahaffy RE, Pollard TD. (2008). Influence of phalloidin on the formation of actin filament branches by Arp2/3 complex. Biochemistry 47: 6460–6467. Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. (2000). Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol 150: 797–806. Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S. (1999). Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol 1: 136–143. |
RhoA induces actin polymerization at focal adhesions by activating the Dia1 formin and inhibits actin filament disassembly by initiating a signaling cascade that leads to phosphorylation and subsequent inactivation of the ADF/cofilin family of actin filament severing/depolymerizing proteins through the action of LIM kinases (146, 230, 383). Furthermore, RhoA promotes contractility by activating the myosin light-chain kinase through ROCK kinase (364).
146. Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol 173: 383–394, 2006. 230. Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 285: 895–898, 1999. 231. Mahaffy RE, Pollard TD. Influence of phalloidin on the formation of actin filament branches by Arp2/3 complex. Biochemistry 47: 6460–6467, 2008. 364. Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol 150: 797–806, 2000. 383. Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol 1: 136–143, 1999. |
Ohne Hinweis auf eine Übernahme. Schließt die auf der vorangegangenen Seite begonnene Übernahme ab. Im Literaturverzeichnis von Shg findet sich keine Referenz für "Vardouli et al 2005". Beim Versuch, die Referenz [230] zu kopieren, hat sich Shg vertan und stattdessen die unter [231] stehende Referenz übernommen. |
|
| [23.] Shg/Fragment 030 01 - Diskussion Bearbeitet: 2. November 2014, 01:26 Hindemith Erstellt: 28. October 2014, 11:33 (Graf Isolan) | CellMigrationGateway 2010, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Shg |
|
|
| Untersuchte Arbeit: Seite: 30, Zeilen: 1ff. (komplett) |
Quelle: CellMigrationGateway 2010 Seite(n): 1 (Internetquelle), Zeilen: - |
|---|---|
| 1.4 Cell Migration
Cells migrate in response to multiple situations they encounter during their lives. In pathology, production of abnormal migratory signals may induce the migration of the wrong cell type to the wrong place, which may have catastrophic effects on tissue homeostasis and overall health. Some examples include autoimmune syndromes in which immune cells home to certain locations (joints in rheumatoid arthritis, and the CNS in multiple sclerosis are two examples) and destroy the supporting tissue, causing severe damage; or the process of metastasis, in which tumor cells abandon the primary tumor and migrate to distant tissues where they generate secondary tumors. There are different modes of cell migration depending on the cell type and the context in which it is migrating. Cells can move as single entities, and the specifics of their motility depend on several factors, adhesion strength and the type of external migratory signals and cues, mechanical pliability, dimensionality, and the organization of the cellular cytoskeleton. The intrinsic properties of the cell interact with the environment to produce a migratory mode or phenotype. Some tumor cells can move by extending membrane blebs, and their actin cytoskeleton is not very organized, either. They have elaborate cytoskeletal structures and adhesions, and their motion is generally slow. It is worth noting that some cell types can switch between these depending on their environment. Cells can also move in groups, including chains of cells and sheet-like layers. It is generally convenient to parse migration into a useful set of component processes, which are often regulated by the same effectors regardless of the cell type and the mode of migration. These processes include polarization, protrusion and adhesion, translocation of the cell body and retraction of the rear. These processes are coordinated and integrated by extensive transient, signaling networks. |
What is Cell Migration?
[...] Cells migrate in response to multiple situations they encounter during their lives. Some examples include: the need to feed (Dictyostelium again); morphogenetic events that require the mobilization of precursors to generate new structures/layers/organs, sometimes at distant locations (during embryogenesis, organogenesis and regeneration); or the presence of environment cues that inform the cells of the need for their movement to accomplish a larger goal (e.g. wound healing or the immune response). In pathology, production of abnormal migratory signals may induce the migration of the wrong cell type to the wrong place, which may have catastrophic effects on tissue homeostasis and overall health. Some examples include autoimmune syndromes in which immune cells home to certain locations (joints in rheumatoid arthritis, and the CNS in multiple sclerosis are two examples) and destroy the supporting tissue, causing severe damage; or the process of metastasis, in which tumor cells abandon the primary tumor and migrate to distant tissues where they generate secondary tumors. There are different modes of cell migration depending on the cell type and the context in which it is migrating. Cells can move as single entities, and the specifics of their motility depend on several factors, e.g., adhesion strength and the type of substratum (including extracellular matrix ligands and other cells), external migratory signals and cues, mechanical pliability, dimensionality, and the organization of the cellular cytoskeleton. The intrinsic properties of the cell interact with the environment to produce a migratory mode or phenotype. For example, nimble, fast-moving and -turning cells, like immune cells, do not have a highly organized cytoskeleton and tend to adhere weakly; their motion is sometimes termed 'amoeboid'. Some tumor cells can move by extending membrane blebs, and their actin cytoskeleton is not very organized, either. Fibroblasts and epithelial precursors lie at another extreme. They have elaborate cytoskeletal structures and adhesions, and their motion is generally slow. It is worth noting that some cell types can switch between these depending on their environment. Cells can also move in groups, including chains of cells and sheet-like layers. It is generally convenient to parse migration into a useful set of component processes, which are often regulated by the same effectors regardless of the cell type and the mode of migration. These processes include polarization, protrusion and adhesion, translocation of the cell body and retraction of the rear. These processes are coordinated and integrated by extensive transient, signaling networks. |
Ohne Hinweis auf eine Übernahme. |
|
| [24.] Shg/Fragment 017 01 - Diskussion Bearbeitet: 2. November 2014, 01:26 Hindemith Erstellt: 29. October 2014, 13:53 (Graf Isolan) | Fragment, Gesichtet, Gu et al 2009, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Shg |
|
|
| Untersuchte Arbeit: Seite: 17, Zeilen: 1-14 |
Quelle: Gu et al 2009 Seite(n): 1-2, Zeilen: 1: li.Sp.1-2 - re.Sp. 1-3 - 2: li.Sp. 1-9 |
|---|---|
| 1.1.2. Membrane Androgen Receptor
Scientific evidence accumulated in recent year’s [sic] points to the existence of membrane androgen receptors (mARs), triggering rapid, non-genomic signals. Although the exact molecular identity of mAR still remains unknown, non-genomic androgen actions manifested within minutes have been reported in various cell types including macrophages and T cells [Benten WP, et al. 1999; Benten WP, et al. 1999], LNCaP [Kampa M, et al. 2002; Wang Z, et al. 2008], T47D [Kampa M, et al. 2005], MCF7 [Kallergi G, et al. 2007], DU145 [Hatzoglou A, et al. 2005; Papadopoulou N, et al. 2008a; Papadopoulou N, et al 2008b], C6 [Gatson JW, et al. 2006], PC12 [Alexaki VI, et al. 2006] or VSMC cells [Somjen D, et al 2004]. These effects are clearly different from those manifested upon activation of the intracellular androgen receptors (iARs) mediating genomic androgen signals resulting in receptor dimerization, nuclear translocation and subsequent activation of androgen-specific target genes. Alexaki VI, Charalampopoulos I, Kampa M, Nifli AP, Hatzoglou A, Gravanis A, Castanas E. (2006). Activation of membrane estrogen receptors induce pro-survival kinases. J Steroid Biochem Mol Biol. 98:97-110. Benten WP, et al. (1999a). Testosterone signaling through internalizable surface receptors in androgen receptor-free macrophages. Mol Biol Cell10: 3113-3123. Benten WP, et al.(1999b). Functional testosterone receptors in plasma membranes of T cells. Faseb J, 13:123-133. Gatson JW, Kaur P, Singh M. (2006). Dihydrotestosterone differentially modulates the mitogen-activated protein kinase and the phosphoinositide 3-kinase/Akt pathways through the nuclear and novel membrane androgen receptor in C6 cells. Endocrinology 147:2028-2034. Hatzoglou A, Kampa M, Kogia C, Charalampopoulos I, Theodoropoulos PA, Anezinis P, Dambaki C, Papakonstanti EA, Stathopoulos EN, Stournaras C, et al. (2005). Membrane androgen receptor activation induces apoptotic regression of human prostate cancer cells in vitro and in vivo. J Clin Endocrinol Metab, 90: 893-903. Kallergi G, Agelaki S, Markomanolaki H, Georgoulias V, Stournaras C. (2007). Activation of FAK/PI3K/Rac1 signaling controls actin reorganization and inhibits cell motility in human cancer cells. Cell Physiol Biochem, 20:977-986. Kampa M, Papakonstanti EA, Hatzoglou A, Stathopoulos EN, Stournaras C, Castanas E. (2002). The human prostate cancer cell line LNCaP bears functional membrane testosterone receptors that increase PSA secretion and modify actin cytoskeleton. Faseb J, 16:1429-1431. Kampa M, Kogia C, Theodoropoulos PA, Anezinis P, Charalampopoulos I, Papakonstanti EA, Stathopoulos EN, Hatzoglou A, Stournaras C, Gravanis A, Castanas E. (2006) Activation of membrane androgen receptors potentiates the antiproliferative effects of paclitaxel on human prostate cancer cells. Mol Cancer Ther, 5:1342-1351. Papadopoulou N, Charalampopoulos I, Alevizopoulos K, Gravanis A, Stournaras C. (2008 a). Rho/ROCK/Actin signaling regualtes membrane androgen receptor induced apoptosis in prostate cancer cells. Exp Cell Res, 314: 3162-3174. Papadopoulou N, Charalampopoulos I, Anagnostopoulou V, Konstantinidis G, Föller M, Gravanis A, Alevizopoulos K, Lang F, Stournaras C. (2008 b). Membrane androgen receptor activation triggers down-regulation of PI-3K/Akt/NF-kappaB activity and induces apoptotic responses via Bad, FasL and caspase-3 in DU-145 prostate cancer cells. Mol Canc. 7:88. Somjen D, Kohen F, Gayer B, Kulik T, Knoll E, Stern N. (2004). Role of putative membrane receptors in the effect of androgens on human vascular cell growth. J Endocrinol. 180:97-106. Wang Z, Liu L, Hou J, Wen D, Yan C, Pu J, Ouyang J, Pan H. (2008). Rapid membrane effect of testosterone in LNCaP cells. Urol Int.81(3):353-9 |
[Seite 1]
Introduction Scientific evidence accumulated in recent years points to the existence of membrane androgen receptors (mAR), triggering rapid, non-genomic signals. Although the exact molecular identity of mAR still remains unknown, nongenomic androgen actions manifested within minutes [Seite 2] have been reported in various cell types including macrophages and T cells [1,2], LNCaP [3,4], T47D [5], MCF7 [6], DU145 [7-9], C6 [10], PC12 [11] or VSMC cells [12]. These effects are clearly different from those manifested upon activation of the intracellular androgen receptors (iAR) mediating genomic androgen signals resulting in receptor dimerization, nuclear translocation and subsequent activation of androgen-specific target genes (reviewed in [13]). 1. Benten WP, Lieberherr M, Stamm O, Wrehlke C, Guo Z, Wunderlich F: Testosterone signaling through internalizable surface receptors in androgen receptor-free macrophages. Mol Biol Cell 1999, 10:3113-3123. 2. Benten WP, Lieberherr M, Giese G, Wrehlke C, Stamm O, Sekeris CE, Mossmann H, Wunderlich F: Functional testosterone receptors in plasma membranes of T cells. Faseb J 1999, 13:123-133. 3. Kampa M, Papakonstanti EA, Hatzoglou A, Stathopoulos EN, Stournaras C, Castanas E: The human prostate cancer cell line LNCaP bears functional membrane testosterone receptors that increase PSA secretion and modify actin cytoskeleton. Faseb J 2002, 16:1429-1431. 4. Wang Z, Liu L, Hou J, Wen D, Yan C, Pu J, Ouyang J, Pan H: Rapid membrane effect of testosterone in LNCaP cells. Urol Int 2008, 81(3):353-359. 5. Kampa M, Kogia C, Theodoropoulos PA, Anezinis P, Charalampopoulos I, Papakonstanti EA, Stathopoulos EN, Hatzoglou A, Stournaras C, Gravanis A, Castanas E: Activation of membrane androgen receptors potentiates the antiproliferative effects of paclitaxel on human prostate cancer cells. Mol Cancer Ther 2006, 5:1342-1351. 6. Kallergi G, Agelaki S, Markomanolaki H, Georgoulias V, Stournaras C: Activation of FAK/PI3K/Rac1 signaling controls actin reorganization and inhibits cell motility in human cancer cells. Cell Physiol Biochem 2007, 20:977-986. 7. Hatzoglou A, Kampa M, Kogia C, Charalampopoulos I, Theodoropoulos PA, Anezinis P, Dambaki C, Papakonstanti EA, Stathopoulos EN, Stournaras C, Gravanis A, Castanas E: Membrane androgen receptor activation induces apoptotic regression of human prostate cancer cells in vitro and in vivo. J Clin Endocrinol Metab 2005, 90:893-903. 8. Papadopoulou N, Charalampopoulos I, Alevizopoulos K, Gravanis A, Stournaras C: Rho/ROCK/Actin signaling regualtes membrane androgen receptor induced apoptosis in prostate cancer cells. Exp Cell Res 2008, 314:3162-3174. 9. Papadopoulou N, Charalampopoulos I, Anagnostopoulou V, Konstantinidis G, Föller M, Gravanis A, Alevizopoulos K, Lang F, Stournaras C: Membrane androgen receptor activation triggers down-regulation of PI-3K/Akt/NF-kappaB activity and induces apoptotic responses via Bad, FasL and caspase-3 in DU-145 prostate cancer cells. Mol Canc 2008, 7:88. 10. Gatson JW, Kaur P, Singh M: Dihydrotestosterone differentially modulates the mitogen-activated protein kinase and the phosphoinositide 3-kinase/Akt pathways through the nuclear and novel membrane androgen receptor in C6 cells. Endocrinology 2006, 147:2028-2034. 11. Alexaki VI, Charalampopoulos I, Kampa M, Nifli AP, Hatzoglou A, Gravanis A, Castanas E: Activation of membrane estrogen receptors induce pro-survival kinases. J Steroid Biochem Mol Biol 2006, 98:97-110. 12. Somjen D, Kohen F, Gayer B, Kulik T, Knoll E, Stern N: Role of putative membrane receptors in the effect of androgens on human vascular cell growth. J Endocrinol 2004, 180:97-106. 13. Heinlein CA, Chang C: Androgen receptor in prostate cancer. Endocr Rev 2004, 25:276-308. |
Obwohl Shg als Coautor von Gu et al (2009) genannt wird, stammt keine der Formulierungen dieses Artikels von Shg (vgl. die Anmerkungen zu Quelle:Shg/Gu_et_al_2009). Ergo: Übernahme eines Fremdtextes (inkl. aller dort zu findenden Literaturreferenzen) ohne jede Kennzeichnung. |
|
| [25.] Shg/Fragment 042 01 - Diskussion Bearbeitet: 2. November 2014, 01:24 Hindemith Erstellt: 31. October 2014, 22:48 (Graf Isolan) | Fragment, Gesichtet, Gu et al 2009, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Shg |
|
|
| Untersuchte Arbeit: Seite: 42, Zeilen: 1-23 |
Quelle: Gu et al 2009 Seite(n): 4, Zeilen: li.Sp. 47-49 - re.Sp. 1-6, 12 ff. |
|---|---|
| At the age of 9 weeks animals were subjected to three cycles of alternating administration of distilled water containing 30 g/L synthetic dextran sulfate sodium (DSS; molecular mass 5000 Da; Wako Pure Chemical Industries, Led. Japan) for 7 days followed by distilled water for subsequent 14 days after intraperitoneal pretreatment with 20 mg/kg 1, 2-dimethylhydrazine (DMH; Sigma-Aldrich Corp. St.Louis.MO.USA). Group B mice received in addition to the carcinogenic treatment 5 mg/kg testosterone-HSA subcutaneously injected three times per week throughout the study period. All mice were sacrificed at the age of 20 weeks. After death, the entire colorectum from the colorectal junction to the anal verge was examined. Fresh specimens were placed in liquid nitrogen and subsequently stored at -80°C for further analysis. Then, the colon was opened longitudinally, washed with PBS, and divided into three portions (proximal, middle and distal). After macroscopic inspection the colon was fixed in a 40% g/L formaldehyde buffer solution (pH.7.4).
In APC mice, animal experiments were carried out in mice of either sex with mutated apc resulting in spontaneous colon tumor development (APCMin/+) obtained from the Jackson Laboratory (USA). The animals were housed under controlled environmental conditions (22-24°C, 50-70% humidity and a 12-h light/dark cycle). Throughout the study the mice had free access to standard pelleted food (C1000, Altromin, Lage, Germany) and tap water. All animal experiments were conducted according to the German law for the care and welfare of animals and were approved by local authorities. |
Experiments were carried out on 7-week old wild-type Balb/c mice of either sex. The animals were housed under controlled environmental conditions (22-24°C, 50-70% humidity and a 12 h light/dark cycle). Throughout the study the mice had free access to standard pelleted food (C1310, Altromin, Heidenau, Germany) and tap water. All animal experiments were conducted according to the German law for the care and welfare of animals and were approved by local authorities
[...] [...] At the age of 9 weeks animals were subjected to three cycles of alternating administration of distilled water containing 30 g/L synthetic dextran sulfate sodium (DSS; molelucar [sic] mass 5000 Da; Wako Pure Chemical Industries, Led. Japan) for 7 days followed by distilled water for subsequent 14 days after intraperitoneal pretreatment with 20 mg/kg 1, 2-dimethylhydrazine (DMH; Sigma-Aldrich Corp. St.Louis. MO. USA). Group B mice received in addition to the carcinogenic treatment 5 mg/kg testosterone-HSA subcutaneously injected three times per week throughout the study period. All mice were sacrificed at the age of 20 weeks. After death, the entire colorectum from the colorectal junction to the anal verge was examined. Fresh specimens were placed in liquid nitrogen and subsequently stored at -80°C for further analysis. Then, the colon was opened longitudinally, washed with PBS, and divided into three portions (proximal, middle and distal). After macroscopic inspection the colon was fixed in a 40% g/L formaldehyde buffer solution (pH.7.4). |
Obwohl Shg als Coautor von Gu et al (2009) genannt wird, stammt keine der Formulierungen dieses Artikels von Shg (vgl. die Anmerkungen zu Quelle:Shg/Gu_et_al_2009). Ergo: Übernahme eines Fremdtextes ohne jede Kennzeichnung. |
|
| [26.] Shg/Fragment 018 01 - Diskussion Bearbeitet: 1. November 2014, 21:40 Hindemith Erstellt: 22. October 2014, 16:05 (SleepyHollow02) | BauernOpfer, Fragment, Gesichtet, Papadopoulou et al 2009, SMWFragment, Schutzlevel sysop, Shg |
|
|
| Untersuchte Arbeit: Seite: 18, Zeilen: 1 ff. (entire page) |
Quelle: Papadopoulou et al 2009 Seite(n): 57, 58, Zeilen: 57:right col., 6 ff., 58:figures 1, 2 |
|---|---|
| Interestingly, while LNCaP prostate cancer cells express functional iAR, the DU145 cell line expresses either nonfunctional iAR, or is iAR-deficient. Therefore, DU145 cells fail to respond to iAR-regulated androgen treatment.[ Alimirah, F., et al 2006] In this cell model, mAR stimulation by testosterone or T-BSA conjugates induced potent actin reorganization, inhibited cell motility and promoted apoptotic regression. [Papadopoulou N, et al. 2008a] But the signaling pathway is different from LNCaP cells. Specifically, mAR activation bypassed the FAK/ PI-3K signaling pathway, as FAK was shown to be constitutively phosphorylated and mAR stimulation failed to further activate the downstream effectors PI-3K and Rac. An alternative pathway functionally distinct from the FAK/PI-3K/Rac signaling was described. [Papadopoulou N, et al. 2008a] This pathway regulated actin reorganization, the induction of apoptosis and the pro-apoptotic machinery. Indeed, long term down regulation of the pro-survival PI-3K/Akt pathway became evident 12–24 h upon mAR activation as indicated by the significant decrease of the phosphorylation levels of PI-3K and Akt. Furthermore, inhibition of NF-jkB translocation and increased FasL expression were documented, while increased caspase 3 activity was measured [Papadopoulou N, et al 2008b].
Figure 3: mAR signaling in human prostate cancer cells A) Non-genomic mAR signaling operating in iAR positive LNCaP human prostate cancer cells regulating actin redistribution and apoptosis. Solid arrows indicate events that have been experimentally proven. Dashed arrows indicate unidentified possible links. See text for details. B) Early and late mAR signaling operating in iAR deficient DU145 human prostate cancer cells regulating actin redistribution, downstream pro-apoptotic signaling, and migration. Solid arrows [indicate events that have been experimentally proven. Dashed arrows indicate unidentified possible links. See text for details. [Papadopoulou N, et al 2009]] Alimirah, F., Chen, J., Basrawala, Z., Xin, H., and Choubey, D. (2006). DU-145 and PC-3 human prostate cancer cell lines express androgen receptor: implications for the androgen receptor functions and regulation. FEBS. Lett. 580, 2294–2300 Papadopoulou N, Charalampopoulos I, Alevizopoulos K, Gravanis A, Stournaras C. (2008 a). Rho/ROCK/Actin signaling regualtes membrane androgen receptor induced apoptosis in prostate cancer cells. Exp Cell Res, 314: 3162-3174. Papadopoulou N, Charalampopoulos I, Anagnostopoulou V, Konstantinidis G, Föller M, Gravanis A, Alevizopoulos K, Lang F, Stournaras C. (2008 b). Membrane androgen receptor activation triggers down-regulation of PI-3K/Akt/NF-kappaB activity and induces apoptotic responses via Bad, FasL and caspase-3 in DU-145 prostate cancer cells. Mol Canc. 7:88. |
[Page 57]
mAR Signaling in iAR-Deficient DU145 Prostate Cancer Cells mAR expression was also reported in iAR-deficient DU145 prostate cancer cells (21). Interestingly, while LNCaP prostate cancer cells express functional intracellular androgen receptors (iAR), the DU145 cell line expresses either nonfunctional iAR (35), or is iAR-deficient (36, 37). Therefore, DU145 cells fail to respond to iAR-regulated androgen treatment (35). In this cell model, mAR stimulation by testosterone or T-BSA conjugates induced potent actin reorganization, inhibited cell motility and promoted apoptotic regression (21, 34). mAR signaling in DU145 cells was recently analyzed providing some very interesting results. Specifically, mAR activation bypassed the FAK/ PI-3K signaling pathway, as FAK was shown to be constitutively phosphorylated and mAR stimulation failed to further activate the downstream effectors PI-3K and Rac1. An alternative pathway functionally distinct from the FAK/PI-3K/Rac signaling was described. [...]. Functional analysis of the pathway revealed that Rho/ROCK signaling regulated, besides actin reorganization, the induction of apoptosis and the pro-apoptotic machinery. Indeed, long term down regulation of the pro-survival PI-3K/Akt pathway became evident 12–24 h upon mAR activation as indicated by the significant decrease of the phosphorylation levels of PI-3K and Akt. Furthermore, inhibition of NF-jB translocation and increased FasL expression were documented, while increased caspase 3 activity was measured (38). [Page 58] Figure 1. Non-genomic mAR signaling operating in iAR positive LNCaP human prostate cancer cells regulating actin redistribution and apoptosis. Solid arrows indicate events that have been experimentally proven. Dashed arrows indicate unidentified possible links. See text for details. Figure 2. Early and late mAR signaling operating in iAR deficient DU145 human prostate cancer cells regulating actin redistribution, downstream pro-apoptotic signaling, and migration. Solid arrows indicate events that have been experimentally proven. Dashed arrows indicate unidentified possible links. See text for details.
21. Hatzoglou, A., Kampa, M., Kogia, C., Charalampopoulos, I., Theodoropoulos, P. A., Anezinis, P., Dambaki, C., Papakonstanti, E. A., Stathopoulos, E. A., Stournaras, C., Gravanis, A., and Castanas E. (2005) Membrane androgen receptor activation induces apoptotic regression of human prostate cancer cells in vitro and in vivo. J. Clin. Endocrinol. Metab. 90, 893–903. 34. Papadopoulou, N., Charalampopoulos, I., Alevizopoulos, K., Gravanis, A., and Stournaras, C. 2008. Rho/ROCK/Actin signaling regualtes membrane androgen receptor induced apoptosis in prostate cancer cells. Exp. Cell. Res., 314, 3162–3174. 35. Alimirah, F., Chen, J., Basrawala, Z., Xin, H., and Choubey, D. (2006) DU-145 and PC-3 human prostate cancer cell lines express androgen receptor: implications for the androgen receptor functions and regulation. FEBS. Lett. 580, 2294–2300. 36. Stone, K. R., Mickey, D. D., Wunderli, H., Mickey, G. H., and Paulson, D. F. (1978) Isolation of a human prostate carcinoma cell line (DU 145). Int. J. Cancer. 21, 274–281. 37. Mitchell, S., Abel, P., Ware, M., Stamp, G., and Lalani, E. (2000) Phenotypic and genotypic characterization of commonly used human prostatic cell lines. BJU. Int. 85, 932–944. 38. Papadopoulou, N., Charalampopoulos, I., Anagnostopoulou, V., Konstandinidis, G., Foeller, M., Gravanis, A., Alevizopoulos, K., Lang, F., and Stournaras, C. Membrane androgen receptor activation triggers down-regulation of PI-3K/AKT/NF-κB activity and induces apoptotic responses via Bad, FasL and caspase 3 in DU-145 prostate cancer cells, Submitted. |
Although the source is given as reference for the figures, nothing has been marked as a citation. Papadopoulou et al. (2008b) does not contain the copied text. |
|
| [27.] Shg/Fragment 027 01 - Diskussion Bearbeitet: 1. November 2014, 21:27 Singulus Erstellt: 25. October 2014, 17:32 (Graf Isolan) | Fragment, Franke et al 2003, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Shg |
|
|
| Untersuchte Arbeit: Seite: 27, Zeilen: 1-23 |
Quelle: Franke et al 2003 Seite(n): 8989, Zeilen: li.Sp. 57-58 - re.Sp. 1ff. |
|---|---|
| [Multiple studies supporting the role of Akt in apoptosis suppression have] connected Akt to cell death regulation either by demonstrating its downregulation following pro-apoptotic insults, or by using gene-transfer experiments that transduce both activated, anti-apoptotic and inactive, pro-apoptotic mutants of Akt.
Taken together, these observations suggest that Akt may play a critical role both in the function of cancer cells and in the pathogenesis of degenerative diseases. By promoting the cell survival of mutated, damaged or transformed cells even under adverse conditions, Akt can promote cancer cell growth by protecting cells from apoptosis, which would otherwise be eliminated by programmed cell death. To experimentally prove the importance of Akt kinases in oncogenic transformation, in a seminal paper, Peter Vogt and colleagues demonstrated that a transformed cellular phenotype could be reverted to normal when using a cell model for PI3K-dependent oncogenesis as long as dominant-negative mutants of Akt were expressed concomitantly [Aoki et al., 1998]. Akt is also likely to play a significant role in degenerative diseases, where excessive or inappropriate cell death occurs possibly because proper trophic factor support is lacking. The relevance of Akt signaling in neurodegenerative disease is supported by studies that examine its activity and function in Alzheimer's disease models in vitro [Hong and Lee, 1997; Weihl et al., 1999]. A role for Akt has also been suggested in other models of human degenerative diseases, including cardiac failure [Matsui et al., 1999] and other cardiovascular diseases where there is increased and chronic loss of cells [Reed and Paternostro, 1999]. Aoki M, Batista O, Bellacosa A, Tsichlis P and Vogt PK. The akt kinase: molecular determinants of oncogenicity. (1998). Proc. Natl. Acad. Sci. USA, 95, 14950–14955. Hong M and Lee VM. (1997). Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neuronsJ. Biol. Chem. 272, 19547–19553. Matsui T, Li L, del Monte F, Fukui Y, Franke TF, Hajjar RJ and Rosenzweig A. (1999). Adenoviral gene transfer of activated phosphatidylinositol 3'-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation, 100, 2373–2379. Reed JC and Paternostro G. (1999). Postmitochondrial regulation of apoptosis during heart failure. (Proc. Natl. Acad. Sci. USA, 96, 7614–7616. Weihl CC, Ghadge GD, Kennedy SG, Hay N, Miller RJ and Roos RP. (1999). Mutant presenilin-1 induces apoptosis and downregulates Akt/PKB. J. Neurosci., 19, 5360–5369. |
Multiple studies supporting the role of Akt in apoptosis suppression have connected Akt to cell death regulation either by demonstrating its downregulation following pro-apoptotic insults, or by using gene-transfer experiments that transduce both activated, anti-apoptotic and inactive, pro-apoptotic mutants of Akt.
Taken together, these observations suggest that Akt may play a critical role both in the function of cancer cells and in the pathogenesis of degenerative diseases. By promoting the cell survival of mutated, damaged or transformed cells even under adverse conditions, Akt can promote cancer cell growth by protecting cells from apoptosis, which would otherwise be eliminated by programmed cell death. To experimentally prove the importance of Akt kinases in oncogenic transformation, in a seminal paper, Peter Vogt and colleagues demonstrated that a transformed cellular phenotype could be reverted to normal when using a cell model for PI3K-dependent oncogenesis as long as dominant-negative mutants of Akt were expressed concomitantly (Aoki et al., 1998). Akt is also likely to play a significant role in degenerative diseases, where excessive or inappropriate cell death occurs possibly because proper trophic factor support is lacking. The relevance of Akt signaling in neurodegenerative disease is supported by studies that examine its activity and function in Alzheimer’s disease models in vitro (Hong and Lee, 1997; Weihl et al., 1999). A role for Akt has also been suggested in other models of human degenerative diseases, including cardiac failure (Matsui et al., 1999) and other cardiovascular diseases where there is increased and chronic loss of cells (Reed and Paternostro, 1999). Aoki M, Batista O, Bellacosa A, Tsichlis P and Vogt PK. (1998). Proc. Natl. Acad. Sci. USA, 95, 14950–14955. Hong M and Lee VM. (1997). J. Biol. Chem., 272, 19547–19553. Matsui T, Li L, del Monte F, Fukui Y, Franke TF, Hajjar RJ and Rosenzweig A. (1999). Circulation, 100, 2373–2379. Reed JC and Paternostro G. (1999). Proc. Natl. Acad. Sci. USA, 96, 7614–7616. Weihl CC, Ghadge GD, Kennedy SG, Hay N, Miller RJ and Roos RP. (1999). J. Neurosci., 19, 5360–5369. |
Ohne Hinweis auf eine Übernahme. |
|
| [28.] Shg/Fragment 026 20 - Diskussion Bearbeitet: 1. November 2014, 21:23 Singulus Erstellt: 25. October 2014, 14:18 (Graf Isolan) | Fragment, Franke et al 2003, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Shg |
|
|
| Untersuchte Arbeit: Seite: 26, Zeilen: 20-31 |
Quelle: Franke et al 2003 Seite(n): 8989, Zeilen: li.Sp. 40-57 |
|---|---|
| 1.3.3. Apoptosis responses by AKT pathway
Akt is a good candidate for mediating PI3K-dependent cell-survival responses. An important function of activated PI3K in cells is the inhibition of programmed cell death [Yao and Cooper, 1995]. The first evidence to show that Akt acts as an anti-apoptotic signaling molecule was observed in cerebellar granule neurons after trophic factor withdrawal [Dudek et al., 1997], and in fibroblasts after forced expression of c-Myc [Kauffmann-Zeh et al., 1997]. Subsequent work in many laboratories has established the principle role of Akt in the regulation of cell survival in several cell types, consistent with its ubiquitous expression pattern. Akt has been implicated as an anti-apoptotic in many different cell death paradigms, including withdrawal of extracellular signaling factors, oxidative and osmotic stress, irradiation and treatment of cells with chemotherapeutic drugs and ischemic shock [Franke et al., 1997; Downward, 1998]. Downward J. (1998) Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 10:262-267. Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR and Greenberg ME. (1997). Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science, 275, 661–665. Franke TF, Kaplan DR and Cantley LC. (1997). PI3K: downstream AKTion blocks apoptosis. Cell, 88, 435–437. Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J and Evan G. (1997). Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB.(1997). Nature, 385, 544–548. Yao R and Cooper GM. (1995) Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor.Science, 267, 2003–2006. |
Apoptosis suppression by Akt
An important function of activated PI3K in cells is the inhibition of programmed cell death (Yao and Cooper, 1995), and Akt is a good candidate for mediating these PI3K-dependent cell-survival responses. The initial evidence to show that Akt acts as an anti-apoptotic signaling molecule was observed in cerebellar granule neurons after trophic factor withdrawal (Dudek et al., 1997), and in fibroblasts after forced expression of c-Myc (Kauffmann-Zeh et al., 1997). Subsequent work in many laboratories has established the principle role of Akt in the regulation of cell survival in several cell types, consistent with its ubiquitous expression pattern. Akt has been implicated as an anti-apoptotic in many different cell death paradigms, including withdrawal of extracellular signaling factors, oxidative and osmotic stress, irradiation and treatment of cells with chemotherapeutic drugs and ischemic shock (Franke et al., 1997a; Downward, 1998). Downward J. (1998). Curr. Opin. Cell Biol., 10, 262–267. Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR and Greenberg ME. (1997). Science, 275, 661–665. Franke TF, Kaplan DR and Cantley LC. (1997a). Cell, 88, 435–437. Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J and Evan G. (1997). Nature, 385, 544–548. Yao R and Cooper GM. (1995). Science, 267, 2003–2006. |
Ohne Hinweis auf eine Übernahme. |
|
| [29.] Shg/Fragment 017 15 - Diskussion Bearbeitet: 1. November 2014, 21:20 Hindemith Erstellt: 22. October 2014, 15:49 (SleepyHollow02) | BauernOpfer, Fragment, Gesichtet, Papadopoulou et al 2009, SMWFragment, Schutzlevel sysop, Shg |
|
|
| Untersuchte Arbeit: Seite: 17, Zeilen: 15-34 |
Quelle: Papadopoulou et al 2009 Seite(n): 57, Zeilen: left col., 18 ff. |
|---|---|
| In prostate cancer, expression of mAR in human tumor cells was initially reported in iAR positive LNCaP cells [Kampa M, et al 2002] and iAR –deficient DU145 cells [Hatzoglou A, et al 2005]. In LNCaP cells study, mAR activation through testosterone-BSA conjugates induced rapid PSA release, fast actin reorganization and additional cell responses like inhibition of cell growth and induction of apoptosis [Hatzoglou A, et al 2005]. The molecular signaling pathway starts from focal adhesion kinase (FAK). Initially, FAK was rapidly phosphorylated and associated with the p85 subunit of the phosphoinositol-3-Kinase (PI-3K). Following this association, the lipid kinase activity of PI-3K and the tyrosine phosphorylation of its p85 regulatory subunit were significantly induced by mAR stimulation. PI-3K activation was accompanied by the downstream upregulation of the Rho small GTPases Cdc42, Rac1, RhoA and RhoB. Rapid activation of these GTPases resulted in actin cytoskeleton reorganization. Yet again, these effects were specific for mAR because three different steroidal and non-steroidal iAR antagonists failed to block the activation of this rapid signaling pathway [Papakonstanti, E. A., et al. 2003]. From these findings it was concluded that mAR activation induced potent apoptotic regression in LNCaP prostate tumor cells controlled by [Rho/ROCK/actin signaling.]
Kampa M, Papakonstanti EA, Hatzoglou A, Stathopoulos EN, Stournaras C, Castanas E. (2002). The human prostate cancer cell line LNCaP bears functional membrane testosterone receptors that increase PSA secretion and modify actin cytoskeleton. Faseb J, 16:1429-1431. Hatzoglou A, Kampa M, Kogia C, Charalampopoulos I, Theodoropoulos PA, Anezinis P, Dambaki C, Papakonstanti EA, Stathopoulos EN, Stournaras C, et al. (2005). Membrane androgen receptor activation induces apoptotic regression of human prostate cancer cells in vitro and in vivo. J Clin Endocrinol Metab, 90: 893-903. Papakonstanti, E. A., Kampa, M., Castanas, E., and Stournaras, C. (2003). A rapid, nongenomic, signaling pathway regulates the actin reorganization induced by activation of membrane testosterone receptors. Mol. Endocrinol. 17, 870–881. |
Expression of mAR in human tumor cells was initially reported in iAR positive LNCaP cells (9). In this study, mAR activation through testosterone, dihydrotestosterone (DHT) or TBSA conjugates induced rapid PSA release and potent and fast actin reorganization. Subsequent studies analyzed additional cell responses triggered by mAR stimulation including mAR-dependent inhibition of cell growth and induction of apoptosis (21). [...] Analysis of the molecular signaling activated by mAR identified a novel mAR-specific non-genomic pathway operating in LNCaP cells (25, 34). Initially, focal adhesion kinase (FAK) was rapidly phosphorylated and associated with the p85 subunit of the phosphoinositol-3-Kinase (PI-3K). Following this association, the lipid kinase activity of PI-3K and the tyrosine phosphorylation of its p85 regulatory subunit were significantly induced by mAR stimulation. PI-3K activation was accompanied by the downstream upregulation of the Rho small GTPases Cdc42, Rac1, RhoA and RhoB. Rapid activation of these GTPases resulted in actin cytoskeleton reorganization. Yet again, these effects were specific for mAR because three different steroidal and non-steroidal iAR antagonists failed to block the activation of this rapid signaling pathway (25). [...] From these findings it was concluded that mAR activation induces potent apoptotic regression in LNCaP prostate tumor cells controlled by Rho/ROCK/actin signaling.
9. Kampa, M., Papakonstanti, E. A., Hatzoglou, A., Stathopoulos, E. N., Stournaras, C., and Castanas, E. (2002) The human prostate cancer cell line LNCaP bears functional membrane testosterone receptors that increase PSA secretion and modify actin cytoskeleton. Faseb. J. 16, 1429–1431. 21. Hatzoglou, A., Kampa, M., Kogia, C., Charalampopoulos, I., Theodoropoulos, P. A., Anezinis, P., Dambaki, C., Papakonstanti, E. A., Stathopoulos, E. A., Stournaras, C., Gravanis, A., and Castanas E. (2005) Membrane androgen receptor activation induces apoptotic regression of human prostate cancer cells in vitro and in vivo. J. Clin. Endocrinol. Metab. 90, 893–903. 25. Papakonstanti, E. A., Kampa, M., Castanas, E., and Stournaras, C. (2003) A rapid, nongenomic, signaling pathway regulates the actin reorganization induced by activation of membrane testosterone receptors. Mol. Endocrinol. 17, 870–881. 34. Papadopoulou, N., Charalampopoulos, I., Alevizopoulos, K., Gravanis, A., and Stournaras, C. 2008. Rho/ROCK/Actin signaling regualtes membrane androgen receptor induced apoptosis in prostate cancer cells. Exp. Cell. Res., 314, 3162–3174. |
The source is given on the next page as reference for a figure. |
|
| [30.] Shg/Fragment 080 24 - Diskussion Bearbeitet: 1. November 2014, 21:20 Singulus Erstellt: 25. October 2014, 12:02 (Graf Isolan) | Fragment, Gesichtet, SMWFragment, Schutzlevel sysop, Shg, Verschleierung, Ziegler et al 2006 |
|
|
| Untersuchte Arbeit: Seite: 80, Zeilen: 24-29 |
Quelle: Ziegler et al 2006 Seite(n): 453, Zeilen: li.Sp. 11-15; re.Sp. 1-4 |
|---|---|
| According to this model vinculin stabilizes focal adhesions and thereby suppresses cell migration, an effect that is relieved by modifications of inositol phospholipids [Holgren C, et al.2010]. Although the precise role of vinculin in focal adhesions remains to be elucidated, recent experimental evidence suggest that vinculin overexpression reduces cell migration, whereas vinculin downregulation enhances cell motility [Holgren C, et al.2010].
Holgren C, Dougherty U, Edwin F, Cerasi D, Taylor I, Fichera A, Joseph L, Bissonnette M, Khare S.(2010) Sprouty-2 controls c-Met expression and metastatic potential of colon cancer cells: sprouty/c-Met upregulation in human colonic adenocarcinomas. Oncogene. 2010.264. |
The picture that emerges is one in which vinculin stabilizes focal adhesions and thereby suppresses cell migration, an effect that is relieved by transient changes in the local concentrations of inositol phospholipids. [...]
[...] Despite the extensive literature on vinculin, its precise role in focal adhesions remains to be elucidated. Vinculin overexpression reduces cell migration, whereas vinculin downregulation enhances cell motility. |
Ohne Hinweis auf eine Übernahme. (In "Holgren C, et al. 2010" findet sich keine der benutzten Formulierungen.) Wegen relativer Kürze käme hier vielleicht auch eine Einordnung als "kW" in Betracht; erstaunlich ist gleichwohl die Textähnlichkeit im Diskussionsteil der Arbeit, zumal durch die Angabe der Fundstelle Holgren der Eindruck entsteht, hier sei sorgfältig referenziert worden. |
|
| [31.] Shg/Fragment 032 06 - Diskussion Bearbeitet: 1. November 2014, 21:18 Singulus Erstellt: 24. October 2014, 22:52 (Graf Isolan) | Fragment, Gesichtet, SMWFragment, Schutzlevel sysop, Shg, Verschleierung, Ziegler et al 2006 |
|
|
| Untersuchte Arbeit: Seite: 32, Zeilen: 6-21 |
Quelle: Ziegler et al 2006 Seite(n): 453, 458, Zeilen: 453: li.Sp. 1-5; 458: re.Sp. 1-16.21-24 |
|---|---|
| 1.4.2. Vinculin and cytoskeleton protein Actin in cell migration
Vinculin is a ubiquitously expressed actin-binding protein. It [sic!] used as a marker for both cell–cell and cell–extracellular matrix (focal adhesion) adherens-type junctions, but its function has remained elusive. A variety of phenotypes of Vinculin-null cells have been shown that the role for vinculin include cell adhesion, cell spreading, focal adhesion stability and strengthening, cell migration and resistance to apoptosis. Vinculin regulate the focal adhesion dynamics, and that transient increases in local phosphoinositide levels. This effect inhibits the vinculin–F-actin interaction, promote focal adhesion turnover and cell motility. Interestingly, the muscle-specific splice variant of vinculin called metavinculin (which contains a 68 amino acid insert in the Vt domain), is localized in dense plaques and costameres, cell–extracellular matrix junctions that are much longer lived than focal adhesions. It could be significant that the Vt/D5 domain of metavinculin interacts less strongly with acidic phospholipids than does the Vt/D5 domain of vinculin [S. Witt et al., 2004]. The association of metavinculin–vinculin heterodimers with F-actin might therefore be relatively resistant to phospholipid competition. Witt S, Zieseniss A, Fock U, Jockusch BM, Illenberger S. (2004). Comparative biochemical analysis suggests that vinculin and metavinculin cooperate in muscular adhesion sites, J. Biol. Chem. 279 pp. 31533–31543. |
[Seite 453]
Vinculin is a ubiquitously expressed actin-binding protein frequently used as a marker for both cell–cell and cell–extracellular matrix (focal adhesion) adherens-type junctions, but its function has remained elusive. [...]
These results suggest a model in which focal adhesion dynamics is regulated by vinculin, and that transient increases in local phosphoinositide levels, which inhibit the vinculin–F-actin interaction, promote focal adhesion turnover and cell motility (Figure 2c). Interestingly, the muscle-specific splice variant of vinculin called metavinculin (which contains a 68 amino acid insert in the Vt domain), is localized in dense plaques and costameres, cell–extracellular matrix junctions that are much longer lived than focal adhesions. It could be significant that the Vt/D5 domain of metavinculin interacts less strongly with acidic phospholipids than does the Vt/D5 domain of vinculin [65]. The association of metavinculin–vinculin heterodimers with F-actin might therefore be relatively resistant to phospholipid competition, resulting in more persistent adhesions. Conclusions [...] Vinculin-null cells show a variety of phenotypes that clearly indicate a role for vinculin in cell adhesion, cell spreading, focal adhesion stability and strengthening, cell migration and resistance to apoptosis. 65 Witt, S. et al. (2004) Comparative biochemical analysis suggests that vinculin and metavinculin cooperate in muscular adhesion sites. J. Biol. Chem. 279, 31533–31543 |
Ohne Hinweis auf eine Übernahme. Beim leicht anpassenden Kopieren ist ein Fehler im Satzbau entstanden, als das eigentlich erforderliche is weggelassen wurde. |
|
| [32.] Shg/Fragment 034 01 - Diskussion Bearbeitet: 1. November 2014, 21:16 Singulus Erstellt: 24. October 2014, 13:38 (Graf Isolan) | Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Shg, Vicente-Manzanares et al 2009 |
|
|
| Untersuchte Arbeit: Seite: 34, Zeilen: 1-12 |
Quelle: Vicente-Manzanares et al 2009 Seite(n): 201-202, Zeilen: 201: re.Sp. 29-44 - 202: li.Sp. 1 ff. |
|---|---|
| [The direct interaction of focal adhesion kinase (FAK) and vinculin with the Arp2/3 complex [DeMali et al., 2002; Serrels et al., 2007], the main] nucleator of actin branching and polymerization in lamellipodia, constitutes a possible mechanism for targeting vinculin and FAK to future adhesion sites. The presence of activated integrins in regions of protrusion outside adhesions suggests that they enter the forming adhesion in an activated state [Galbraith et al., 2007; Kiosses et al., 2001]. The other implication is that adhesions might nucleate actin polymerization. This would provide a mechanism for the formation of actin filaments on which adhesions elongate; these appear to elongate from nascent adhesions at the lamellipodium-lamellum interface. This possibility is supported by the observation that purified integrin-adhesion complexes have actin-polymerization activity [Butler et al., 2006]. Although the neutralization of Arp2/3 in β3-integrin-containing adhesion complexes did not impair actin polymerization, targeting of the formin mDia did [Butler et al., 2006].
Butler, B., Gao, C., Mersich, A. T. and Blystone, S. D. (2006). Purified integrin adhesion complexes exhibit actin-polymerization activity. Curr. Biol. 16, 242-251. DeMali, K. A., Barlow, C. A. and Burridge, K. (2002). Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J. Cell Biol. 159, 881-891. Galbraith, C. G., Yamada, K. M. and Galbraith, J. A. (2007). Polymerizing actin fibers position integrins primed to probe for adhesion sites. Science 315, 992-995. Kiosses, W. B., Shattil, S. J., Pampori, N. and Schwartz, M. A. (2001). Rac recruits high-affinity integrin alphavbeta3 to lamellipodia in endothelial cell migration. Nat. Cell Biol. 3, 316-320. Serrels, B., Serrels, A., Brunton, V. G., Holt, M., McLean, G. W., Gray, C. H., Jones, G. E. and Frame, M. C. (2007). Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat. Cell Biol. 9, 1046-1056. |
[Seite 201]
In this context, the direct interaction of focal adhesion kinase (FAK) and vinculin with the Arp2/3 complex (DeMali et al., 2002; Serrels et al., 2007), the main nucleator of actin branching and polymerization in lamellipodia, constitutes a possible mechanism for targeting vinculin and FAK to future adhesion sites. The presence of activated integrins in regions of protrusion outside adhesions suggests that they enter the forming adhesion in an activated state (Galbraith et al., 2007; Kiosses et al., 2001). The other implication is that adhesions might nucleate actin polymerization. This would provide a mechanism for the formation of actin filaments on which adhesions elongate; these appear to elongate from nascent adhesions at the lamellipodium-lamellum interface. This possibility is supported by the observation that purified integrin-adhesion complexes have actin-polymerization activity (Butler et al., 2006). Although the neutralization of Arp2/3 in [Seite 202] β3-integrin-containing adhesion complexes did not impair actin polymerization, targeting of the formin mDia did (Butler et al., 2006). Butler, B., Gao, C., Mersich, A. T. and Blystone, S. D. (2006). Purified integrin adhesion complexes exhibit actin-polymerization activity. Curr. Biol. 16, 242-251. DeMali, K. A., Barlow, C. A. and Burridge, K. (2002). Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J. Cell Biol. 159, 881-891. Galbraith, C. G., Yamada, K. M. and Galbraith, J. A. (2007). Polymerizing actin fibers position integrins primed to probe for adhesion sites. Science 315, 992-995. Kiosses, W. B., Shattil, S. J., Pampori, N. and Schwartz, M. A. (2001). Rac recruits high-affinity integrin alphavbeta3 to lamellipodia in endothelial cell migration. Nat. Cell Biol. 3, 316-320. Serrels, B., Serrels, A., Brunton, V. G., Holt, M., McLean, G. W., Gray, C. H., Jones, G. E. and Frame, M. C. (2007). Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat. Cell Biol. 9, 1046-1056. |
Art und Umfang der Übernahme bleiben ungekennzeichnet. |
|
| [33.] Shg/Fragment 033 23 - Diskussion Bearbeitet: 1. November 2014, 15:26 Singulus Erstellt: 24. October 2014, 11:17 (Graf Isolan) | Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Shg, Vicente-Manzanares et al 2009 |
|
|
| Untersuchte Arbeit: Seite: 33, Zeilen: 23-32 |
Quelle: Vicente-Manzanares et al 2009 Seite(n): 201, 202, Zeilen: 201: re.Sp. 25-34; 202: li.Sp. 3-9 |
|---|---|
| Actin polymerization and adhesion formation are linked. Actin polymerization determines the rate of adhesion assembly and potentially nucleates adhesions that contain activated integrins; conversely, adhesions provide nucleation points that may support actin polymerization. Adhesions and actin are also physically linked and this linkage coordinates adhesion assembly and disassembly and the processes they regulate. Adhesion assembly requires actin polymerization suggesting that the interaction of a subset of adhesion components with actin nucleates the nascent adhesion, which is then stabilized by its association with integrins. The direct interaction of focal adhesion kinase (FAK) and vinculin with the Arp2/3 complex [DeMali et al., 2002; Serrels et al., 2007], the main [nucleator of actin branching and polymerization in lamellipodia, constitutes a possible mechanism for targeting vinculin and FAK to future adhesion sites.]
DeMali, K. A., Barlow, C. A. and Burridge, K. (2002). Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J. Cell Biol. 159, 881-891. Serrels, B., Serrels, A., Brunton, V. G., Holt, M., McLean, G. W., Gray, C. H., Jones, G. E. and Frame, M. C. (2007). Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat. Cell Biol. 9, 1046-1056. |
[Seite 201]
However, the observation that adhesion assembly requires actin polymerization suggests that the interaction of a subset of adhesion components with actin nucleates the nascent adhesion, which is then stabilized by its association with integrins. In this context, the direct interaction of focal adhesion kinase (FAK) and vinculin with the Arp2/3 complex (DeMali et al., 2002; Serrels et al., 2007), the main nucleator of actin branching and polymerization in lamellipodia, constitutes a possible mechanism for targeting vinculin and FAK to future adhesion sites. [Seite 202] Thus, actin polymerization and adhesion formation are linked. Actin polymerization determines the rate of adhesion assembly and potentially nucleates adhesions that contain activated integrins; conversely, adhesions provide nucleation points that may support actin polymerization. Adhesions and actin are also physically linked and this linkage coordinates adhesion assembly and disassembly and the processes they regulate. DeMali, K. A., Barlow, C. A. and Burridge, K. (2002). Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J. Cell Biol. 159, 881-891. Serrels, B., Serrels, A., Brunton, V. G., Holt, M., McLean, G. W., Gray, C. H., Jones, G. E. and Frame, M. C. (2007). Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat. Cell Biol. 9, 1046-1056. |
Ohne Hinweis auf eine Übernahme. |
|
| [34.] Shg/Fragment 028 01 - Diskussion Bearbeitet: 1. November 2014, 13:43 Singulus Erstellt: 23. October 2014, 12:17 (Graf Isolan) | Danial 2009, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Shg |
|
|
| Untersuchte Arbeit: Seite: 28, Zeilen: 1 ff. (komplett) |
Quelle: Danial 2009 Seite(n): S55, S56, S57, Zeilen: S55:li.Sp. 5-18; S56:re.Sp. 9-33.; S57:re.Sp. 1-11 |
|---|---|
| [Although signals transduced by the engagement of growth factor receptors, such as cytokines, insulin-like-growth] factor (IGF) and nerve growth factor (NGF), were long known to promote survival, the molecular mechanisms linking their survival-promoting effect to the direct inhibition of apoptosis emerged with the identification of select components of the core apoptotic machinery that are modulated by phosphorylation events downstream of survival signaling [Datta et al., 1999; Amaravadi and Thompson, 2005]. BAD's capacity to bind and neutralize its anti-apoptotic partners, BCL-2, BCL-XL and BCL-W, is inhibited on phosphorylation by survival kinases activated by trophic factors. Various kinases have been shown to phosphorylate BAD. S136 is a preferred substrate for AKT and p70S6 kinases in the PI3K signaling pathway [Datta et al., 1997; Del Peso et al., 1997; Blume-Jensen et al., 1998; Eves et al., 1998; Harada et al., 2001]. S112 and S155 harbor bona fide protein kinase A (PKA) consensus sites that can also be recognized by p90 ribosomal S6 kinase (p90RSK), a kinase activated by the MAPK pathway that shares multiple common substrates with PKA [Datta et al., 2000; Tan et al., 2000; Houslay, 2006]. Modification of BAD by p90RSK is consistent with several studies indicating that the activation of the RAS/RAF/MEK/MAPK pathway modulates BAD phosphorylation [Bonni et al., 1999; Fang et al., 1999; Scheid et al., 1999]. RAF can localize to mitochondria [Wang et al., 1996; Gotz et al., 2005], and its activated forms promote BAD phosphorylation [Fang et al., 1999]. However, RAF modulation of BAD phosphorylation is likely indirect through other kinases such as AKT [Fang et al., 1999; Wiese et al., 2001; von Gise et al., 2001; Gotz et al., 2005]. PIM kinases constitute another class of survival kinases that phosphorylate BAD predominantly on the S112 site [Fox et al., 2003; Yan et al., 2003; Macdonald et al., 2006]. The interrelationship of these phosphorylation events is especially intriguing as it suggests that BAD modification may serve as a node where distinct signaling pathways converge to regulate the core apoptotic machinery. Recent studies have proposed a sequential model of BAD dephosphorylation initiated by pS112 dephosphorylation, which may then expose pS136 and pS155 residues for dephosphorylation [Chiang et al., 2003]. Thus, both phosphorylation and dephosphorylation of BAD at the three serine sites seem to be tiered processes. Although S136 is the apical serine the phosphorylation of which is needed for neutralizing BAD's apoptotic function, pS112 dephosphorylation may be the initial dephosphorylation event required for [promoting the apoptotic activity of BAD.]
Amaravadi R, Thompson CB. (2005). The survival kinases Akt and Pim as potential pharmacological targets. J Clin Invest 115: 2618–2624. Blume-Jensen P, Janknecht R, Hunter T. (1998). The kit receptor promotes cell survival via activation of PI 3-kinase and subsequent Akt-mediated phosphorylation of Bad on Ser136. Curr Biol 8: 779–782. Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. (1999). Cell survival promoted by the Ras–MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286: 1358–1362. Chiang CW, Kanies C, Kim KW, Fang WB, Parkhurst C, Xie M et al. (2003). Protein phosphatase 2A dephosphorylation of phosphoserine 112 plays the gatekeeper role for BAD-mediated apoptosis. Mol Cell Biol 23: 6350–6362. Datta SR, Brunet A, Greenberg ME. (1999). Cellular survival: a play in three Akts. Genes Dev 13: 2905–2927. Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. (1997) Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 91:231-241. Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB et al. (2000). 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell 6: 41–51. del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. (1997). Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 24;278(5338):687-9. Eves EM, Xiong W, Bellacosa A, Kennedy SG, Tsichlis PN, Rosner MR et al. (1998). Akt, a target of phosphatidylinositol 3-kinase, inhibits apoptosis in a differentiating neuronal cell line. Mol Cell Biol 18: 2143–2152. Fang X, Yu S, Eder A, Mao M, Bast Jr RC, Boyd D et al. (1999). Regulation of BAD phosphorylation at serine 112 by the Ras-mitogen-activated protein kinase pathway. Oncogene 18: 6635–6640. Fox CJ, Hammerman PS, Cinalli RM, Master SR, Chodosh LA, Thompson CB. (2003). The serine/threonine kinase Pim-2 is a transcriptionally regulated apoptotic inhibitor. Genes Dev 17: 1841–1854. Gotz R, Wiese S, Takayama S, Camarero GC, Rossoll W, Schweizer U et al. (2005). Bag1 is essential for differentiation and survival of hematopoietic and neuronal cells. Nat Neurosci 8: 1169–1178. Harada H, Andersen JS, Mann M, Terada N, Korsmeyer SJ. (2001). p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc Natl Acad Sci USA 98: 9666–9670. Houslay MD. (2006). A RSK(y) relationship with promiscuous PKA. Sci STKE 2006: pe32. Macdonald A, Campbell DG, Toth R, McLauchlan H, Hastie CJ, Arthur JS. (2006). Pim kinases phosphorylate multiple sites on Bad and promote 14-3-3 binding and dissociation from Bcl-XL. BMC Cell Biol 7: 1. Scheid MP, Schubert KM, Duronio V. (1999). Regulation of bad phosphorylation and association with Bcl-x(L) by the MAPK/Erk kinase. J Biol Chem 274: 31108–31113. Tan Y, Demeter MR, Ruan H, Comb MJ. (2000). BAD Ser-155 phosphorylation regulates BAD/Bcl-XL interaction and cell survival. J Biol Chem 275: 25865–25869. von Gise A, Lorenz P, Wellbrock C, Hemmings B, Berberich-Siebelt F, Rapp UR et al. (2001). Apoptosis suppression by Raf-1 and MEK1 requires MEK- and phosphatidylinositol 3-kinase-dependent signals. Mol Cell Biol 21: 2324–2336. Wiese S, Pei G, Karch C, Troppmair J, Holtmann B, Rapp UR et al. (2001). Specific function of B-Raf in mediating survival of embryonic motoneurons and sensory neurons. Nat Neurosci 4: 137–142. Yan B, Zemskova M, Holder S, Chin V, Kraft A, Koskinen PJ et al. (2003). The PIM-2 kinase phosphorylates BAD on serine 112 and reverses BAD-induced cell death. J Biol Chem 278: 45358–45367. |
[Seite S55]
Although signals transduced by the engagement of growth factor receptors, such as cytokines, insulin-like-growth factor (IGF) and nerve growth factor (NGF), were long known to promote survival, the molecular mechanisms linking their survival-promoting effect to the direct inhibition of apoptosis emerged with the identification of select components of the core apoptotic machinery that are modulated by phosphorylation events downstream of survival signaling (Datta et al., 1999; Amaravadi and Thompson, 2005). BAD’s capacity to bind and neutralize its anti-apoptotic partners, BCL-2, BCL-XL and BCL-W, is inhibited on phosphorylation by survival kinases activated by trophic factors (Figure 2a). [Seite S56] BAD kinases Depending on the cellular context, various kinases have been shown to phosphorylate BAD (Figure 3). S136 is a preferred substrate for AKT and p70S6 kinases in the PI3K signaling pathway (Datta et al., 1997; del Peso et al., 1997; Blume-Jensen et al., 1998; Eves et al., 1998; Harada et al., 2001). S112 and S155 harbor bona fide protein kinase A (PKA) consensus sites that can also be recognized by p90 ribosomal S6 kinase (p90RSK), a kinase activated by the MAPK pathway that shares multiple common substrates with PKA (Datta et al., 2000; Tan et al., 2000; Houslay, 2006). Modification of BAD by p90RSK is consistent with several studies indicating that the activation of the RAS/RAF/MEK/MAPK pathway modulates BAD phosphorylation (Bonni et al., 1999; Fang et al., 1999; Scheid et al., 1999). RAF can localize to mitochondria (Wang et al., 1996a; Gotz et al., 2005), and its activated forms promote BAD phosphorylation (Fang et al., 1999). However, RAF modulation of BAD phosphorylation is likely indirect through other kinases such as AKT (Fang et al., 1999; Wiese et al., 2001; von Gise et al., 2001; Gotz et al., 2005). PIM kinases constitute another class of survival kinases that phosphorylate BAD predominantly on the S112 site (Fox et al., 2003; Yan et al., 2003; Macdonald et al., 2006). [...] The interrelationship of these phosphorylation events is especially intriguing as it suggests that BAD modification may serve as a node where distinct signaling pathways converge to regulate the core apoptotic machinery. [Seite S57] Recent studies have proposed a sequential model of BAD dephosphorylation initiated by pS112 dephosphorylation, which may then expose pS136 and pS155 residues for dephosphorylation (Chiang et al., 2003). Thus, both phosphorylation and dephosphorylation of BAD at the three serine sites seem to be tiered processes. Although S136 is the apical serine the phosphorylation of which is needed for neutralizing BAD’s apoptotic function, pS112 dephosphorylation may be the initial dephosphorylation event required for promoting the apoptotic activity of BAD. Amaravadi R, Thompson CB. (2005). The survival kinases Akt and Pim as potential pharmacological targets. J Clin Invest 115: 2618–2624. Blume-Jensen P, Janknecht R, Hunter T. (1998). The kit receptor promotes cell survival via activation of PI 3-kinase and subsequent Akt-mediated phosphorylation of Bad on Ser136. Curr Biol 8: 779–782. Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. (1999). Cell survival promoted by the Ras–MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286: 1358–1362. Chiang CW, Kanies C, Kim KW, Fang WB, Parkhurst C, Xie M et al. (2003). Protein phosphatase 2A dephosphorylation of phosphoserine 112 plays the gatekeeper role for BAD-mediated apoptosis. Mol Cell Biol 23: 6350–6362. Datta SR, Brunet A, Greenberg ME. (1999). Cellular survival: a play in three Akts. Genes Dev 13: 2905–2927. Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y et al. (1997). Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91: 231–241. Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB et al. (2000). 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell 6: 41–51. del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. (1997). Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278: 687–689. Eves EM, Xiong W, Bellacosa A, Kennedy SG, Tsichlis PN, Rosner MR et al. (1998). Akt, a target of phosphatidylinositol 3-kinase, inhibits apoptosis in a differentiating neuronal cell line. Mol Cell Biol 18: 2143–2152. Fang X, Yu S, Eder A, Mao M, Bast Jr RC, Boyd D et al. (1999). Regulation of BAD phosphorylation at serine 112 by the Ras-mitogen-activated protein kinase pathway. Oncogene 18: 6635–6640. Fox CJ, Hammerman PS, Cinalli RM, Master SR, Chodosh LA, Thompson CB. (2003). The serine/threonine kinase Pim-2 is a transcriptionally regulated apoptotic inhibitor. Genes Dev 17: 1841–1854. Gotz R, Wiese S, Takayama S, Camarero GC, Rossoll W, Schweizer U et al. (2005). Bag1 is essential for differentiation and survival of hematopoietic and neuronal cells. Nat Neurosci 8: 1169–1178. Harada H, Andersen JS, Mann M, Terada N, Korsmeyer SJ. (2001). p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc Natl Acad Sci USA 98: 9666–9670. Houslay MD. (2006). A RSK(y) relationship with promiscuous PKA. Sci STKE 2006: pe32. Macdonald A, Campbell DG, Toth R, McLauchlan H, Hastie CJ, Arthur JS. (2006). Pim kinases phosphorylate multiple sites on Bad and promote 14-3-3 binding and dissociation from Bcl-XL. BMC Cell Biol 7: 1. Scheid MP, Schubert KM, Duronio V. (1999). Regulation of bad phosphorylation and association with Bcl-x(L) by the MAPK/Erk kinase. J Biol Chem 274: 31108–31113. Tan Y, Demeter MR, Ruan H, Comb MJ. (2000). BAD Ser-155 phosphorylation regulates BAD/Bcl-XL interaction and cell survival. J Biol Chem 275: 25865–25869. von Gise A, Lorenz P, Wellbrock C, Hemmings B, Berberich-Siebelt F, Rapp UR et al. (2001). Apoptosis suppression by Raf-1 and MEK1 requires MEK- and phosphatidylinositol 3-kinase-dependent signals. Mol Cell Biol 21: 2324–2336. Wang HG, Rapp UR, Reed JC. (1996a). Bcl-2 targetsthe protein kinase Raf-1 to mitochondria. Cell 87: 629–638. Wiese S, Pei G, Karch C, Troppmair J, Holtmann B, Rapp UR et al. (2001). Specific function of B-Raf in mediating survival of embryonic motoneurons and sensory neurons. Nat Neurosci 4: 137–142. Yan B, Zemskova M, Holder S, Chin V, Kraft A, Koskinen PJ et al. (2003). The PIM-2 kinase phosphorylates BAD on serine 112 and reverses BAD-induced cell death. J Biol Chem 278: 45358–45367. |
Sieht nach einem sorgfältigen und sehr detaillierten Literaturreferat aus - ist aber eine Kopie bis hin zur äußeren Form der Literaturverweise - ohne Hinweis auf eine Übernahme. "Gotz et al., 2005" müsste "Götz et al., 2005" sein (in beiden Texten). Der Nachweis für "Wang et al., 1996" fehlt bei Shg. |
|
| [35.] Shg/Fragment 029 01 - Diskussion Bearbeitet: 1. November 2014, 09:36 SleepyHollow02 Erstellt: 24. October 2014, 10:48 (Graf Isolan) | Danial 2009, Fragment, Gesichtet, KomplettPlagiat, SMWFragment, Schutzlevel sysop, Shg |
|
|
| Untersuchte Arbeit: Seite: 29, Zeilen: 1-3 |
Quelle: Danial 2009 Seite(n): S57, Zeilen: re.Sp. 7-14 |
|---|---|
| [Although S136 is the apical serine the phosphorylation of which is needed for neutralizing BAD's apoptotic function, pS112 dephosphorylation may be the initial dephosphorylation event required for] promoting the apoptotic activity of BAD. It is also possible that, in addition to directly targeting specific serine sites, BAD phosphatases may inactivate the survival kinases [Andjelkovic et al., 1996; Djouder et al., 2007].
Andjelkovic M, Jakubowicz T, Cron P, Ming XF, Han JW, Hemmings BA. (1996). Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc Natl Acad Sci USA 93: 5699–5704. Djouder N, Metzler SC, Schmidt A, Wirbelauer C, Gstaiger M, Aebersold R et al. (2007). S6K1-mediated disassembly of mitochondrial URI/PP1gamma complexes activates a negative feedback program that counters S6K1 survival signaling. Mol Cell 28: 28–40. |
Although S136 is the apical serine the phosphorylation of which is needed for neutralizing BAD’s apoptotic function, pS112 dephosphorylation may be the initial dephosphorylation event required for promoting the apoptotic activity of BAD. It is also possible that, in addition to directly targeting specific serine sites, BAD phosphatases may inactivate the survival kinases (Andjelkovic et al., 1996; Djouder et al., 2007).
Andjelkovic M, Jakubowicz T, Cron P, Ming XF, Han JW, Hemmings BA. (1996). Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc Natl Acad Sci USA 93: 5699–5704. Djouder N, Metzler SC, Schmidt A, Wirbelauer C, Gstaiger M, Aebersold R et al. (2007). S6K1-mediated disassembly of mitochondrial URI/PP1gamma complexes activates a negative feedback program that counters S6K1 survival signaling. Mol Cell 28: 28–40. |
Ohne Hinweis auf eine Übernahme. Schließt die auf der vorangegangenen Seite begonnene Kopie der Vorlage ab. |
|